Abstract
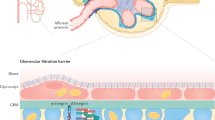
The unique permeability characteristics of the glomerular capillary wall depend on its three-layer structure, consisting of endothelial cells, the basement membrane and podocytes. These components form the glomerular filtration barrier (GFB). That albuminuria may occur in the absence of changes in podocyte foot processes suggests that GFB components other than podocytes have essential roles in albumin handling. The endothelium forms the first part of the GFB and is characterized by fenestrations—transcellular holes that are filled with endothelial glycocalyx, a hydrated mesh principally comprised of proteoglycans. The glycocalyx and adsorbed plasma constituents form the endothelial surface layer (ESL). Human and animal studies have shown that the glomerular ESL restricts macromolecule passage and ensures that plasma albumin is largely excluded from the GFB. The glomerular endothelium is also likely to indirectly influence glomerular albumin handling by modifying podocyte behaviour. These modifications may occur physiologically through soluble mediators and/or pathologically through increased exposure of podocytes to plasma components as a consequence of ESL dysfunction. The importance of the glomerular endothelium and ESL in albumin handling also sheds light on the relationship between albuminuria and vascular disease. The therapeutic potential that this relationship offers will become evident with better understanding of the structure, composition and regulation of the glycocalyx.
Key Points
-
The glomerular filtration barrier (GFB) functions as a whole with each layer making an important contribution
-
Albuminuria can occur in the absence of changes in podocyte foot processes, confirming that other GFB components have essential roles in albumin handling
-
The glomerular endothelium is characterized by fenestrations (transcellular holes essential for filtration function) and a surface glycocalyx that extends over the fenestrations
-
The glomerular endothelial surface layer (ESL) consists of a surface-bound glycocalyx and adsorbed plasma components; dysfunction of the ESL contributes to increased glomerular permeability in disease
-
Cell-to-cell communication has an important role in glomerular homeostasis; glomerular endothelial cell-to-podocyte communication is likely to regulate the podocyte contribution to glomerular albumin handling in health and disease
-
Dysfunction of the glomerular endothelial glycocalyx is an attractive therapeutic target that links albuminuria to systemic vascular disease and potentially helps to explain why albuminuria is a risk factor for cardiovascular disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Neal, C. R., Crook, H., Bell, E., Harper, S. J. & Bates, D. O. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J. Am. Soc. Nephrol. 16, 1223–1235 (2005).
Norden, A. G. et al. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 60, 1885–1892 (2001).
Michel, C. C. & Curry, F. E. Microvascular permeability. Physiol. Rev. 79, 703–761 (1999).
Pavenstadt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Haraldsson, B. & Sorensson, J. Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol. Sci. 19, 7–10 (2004).
Haraldsson, B., Nystrom, J. & Deen, W. M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451–487 (2008).
Ryan, G. B. & Karnovsky, M. J. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 9, 36–45 (1976).
Lund, U. et al. Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am. J. Physiol. Renal Physiol. 284, F1226–F1234 (2003).
Rippe, C., Asgeirsson, D., Venturoli, D., Rippe, A. & Rippe, B. Effects of glomerular filtration rate on Ficoll sieving coefficients (θ) in rats. Kidney Int. 69, 1326–1332 (2006).
Rippe, B. What is the role of albumin in proteinuric glomerulopathies? Nephrol. Dial. Transplant. 19, 1–5 (2004).
Sugimoto, H. et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 278, 12605–12608 (2003).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003).
Yoshioka, T., Ichikawa, I. & Fogo, A. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J. Am. Soc. Nephrol. 2, 902–912 (1991).
Friden, V. et al. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 79, 1322–1330 (2011).
Davis, B. et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J. Am. Soc. Nephrol. 18, 2320–2329 (2007).
Macconi, D. et al. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J. Am. Soc. Nephrol. 11, 477–489 (2000).
Stillman, I. E. & Karumanchi, S. A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol. 18, 2281–2284 (2007).
Pagtalunan, M. E. et al. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Invest. 99, 342–348 (1997).
Branten, A. J., van den Born, J., Jansen, J. L., Assmann, K. J. & Wetzels, J. F. Familial nephropathy differing from minimal change nephropathy and focal glomerulosclerosis. Kidney Int. 59, 693–701 (2001).
van den Berg, J. G., van den Bergh Weerman, M. A., Assmann, K. J., Weening, J. J. & Florquin, S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 66, 1901–1906 (2004).
Kriz, W., Shirato, I., Nagata, M., Lehir, M. & Lemley, K. V. The podocyte's response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 304, F333–F347 (2012).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Satchell, S. C. & Tooke, J. E. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia 51, 714–725 (2008).
Satchell, S. C. & Braet, F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 296, F947–F956 (2009).
Ichimura, K., Stan, R. V., Kurihara, H. & Sakai, T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J. Am. Soc. Nephrol. 19, 1463–1471 (2008).
Deen, W. M., Lazzara, M. J. & Myers, B. D. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 281, F579–F596 (2001).
Satchell, S. C., Anderson, K. L. & Mathieson, P. W. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15, 566–574 (2004).
Satchell, S. C. et al. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 69, 1633–1640 (2006).
Sorensson, J. et al. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J. Am. Soc. Nephrol. 13, 2639–2647 (2002).
Rostgaard, J. & Qvortrup, K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc. Res. 53, 1–13 (1997).
Hjalmarsson, C., Johansson, B. R. & Haraldsson, B. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc. Res. 67, 9–17 (2004).
Dane, M. J. et al. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am. J. Pathol. 182, 1532–1540 (2013).
Levick, J. R. & Smaje, L. H. An analysis of the permeability of a fenestra. Microvasc. Res. 33, 233–256 (1987).
Salmon, A. H. & Satchell, S. C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 226, 562–574 (2012).
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A. & oude Egbrink, M. G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007).
Weinbaum, S., Tarbell, J. M. & Damiano, E. R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007).
Pries, A. R., Secomb, T. W. & Gaehtgens, P. The endothelial surface layer. Pflugers Arch. 440, 653–666 (2000).
Oohira, A., Wight, T. N. & Bornstein, P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J. Biol. Chem. 258, 2014–2021 (1983).
Lindblom, A., Carlstedt, I. & Fransson, L. A. Identification of the core proteins in proteoglycans synthesized by vascular endothelial cells. Biochem. J. 261, 145–153 (1989).
Griesmacher, A., Hennes, R., Keller, R. & Greiling, H. Proteoglycans from human umbilical vein endothelial cells. Eur. J. Biochem. 168, 95–101 (1987).
Avasthi, P. S. & Koshy, V. The anionic matrix at the rat glomerular endothelial surface. Anat. Rec. 220, 258–266 (1988).
Singh, A. et al. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 18, 2885–2893 (2007).
Singh, A. et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am. J. Physiol. Renal Physiol. 300, F40–F48 (2011).
Foster, R. R. et al. Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am. J. Pathol. 183, 604–616 (2013).
Squire, J. M. et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 136, 239–255 (2001).
Arkill, K. P. et al. Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism? Biophys. J. 101, 1046–1056 (2011).
Bankston, P. W. & Milici, A. J. A survey of the binding of polycationic ferritin in several fenestrated capillary beds: indication of heterogeneity in the luminal glycocalyx of fenestral diaphragms. Microvasc. Res. 26, 36–48 (1983).
Salmon, A. H. et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23, 1339–1350 (2012).
Ebong, E. E., Macaluso, F. P., Spray, D. C. & Tarbell, J. M. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler. Thromb. Vasc. Biol. 31, 1908–1915 (2011).
Simionescu, M., Simionescu, N., Silbert, J. E. & Palade, G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J. Cell Biol. 90, 614–621 (1981).
Zeng, Y., Ebong, E. E., Fu, B. M. & Tarbell, J. M. The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS ONE 7, e43168 (2012).
Fried, T. A., McCoy, R. N., Osgood, R. W. & Stein, J. H. Effect of albumin on glomerular ultrafiltration coefficient in isolated perfused dog glomerulus. Am. J. Physiol. 250, F901–F906 (1986).
Schnitzer, J. E. & Pinney, E. Quantitation of specific binding of orosomucoid to cultured microvascular endothelium: role in capillary permeability. Am. J. Physiol. 263, H48–H55 (1992).
Haraldsson, B. S., Johnsson, E. K. & Rippe, B. Glomerular permselectivity is dependent on adequate serum concentrations of orosomucoid. Kidney Int. 41, 310–316 (1992).
Lennon, R. et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J. Am. Soc. Nephrol. 19, 2140–2149 (2008).
Singh, A. et al. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS ONE 8, e55852 (2013).
Jeansson, M. & Haraldsson, B. Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J. Am. Soc. Nephrol. 14, 1756–1765 (2003).
Jeansson, M. & Haraldsson, B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am. J. Physiol. Renal Physiol. 290, F111–F116 (2006).
Gelberg, H., Healy, L., Whiteley, H., Miller, L. A. & Vimr, E. In vivo enzymatic removal of α2 6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab. Invest. 74, 907–920 (1996).
Bakker, W. W. et al. Protease activity of plasma hemopexin. Kidney Int. 68, 603–610 (2005).
Meuwese, M. C. et al. Endothelial surface layer degradation by chronic hyaluronidase infusion induces proteinuria in apolipoprotein E-deficient mice. PLoS ONE 5, e14262 (2010).
Jeansson, M., Bjorck, K., Tenstad, O. & Haraldsson, B. Adriamycin alters glomerular endothelium to induce proteinuria. J. Am. Soc. Nephrol. 20, 114–122 (2009).
Remuzzi, A., Puntorieri, S., Battaglia, C., Bertani, T. & Remuzzi, G. Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J. Clin. Invest. 85, 541–549 (1990).
Kuwabara, A., Satoh, M., Tomita, N., Sasaki, T. & Kashihara, N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 59, 2056–2065 (2010).
Adembri, C. et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit. Care 15, R277 (2011).
Jeansson, M., Granqvist, A. B., Nystrom, J. S. & Haraldsson, B. Functional and molecular alterations of the glomerular barrier in long-term diabetes in mice. Diabetologia 49, 2200–2209 (2006).
Satoh, M. et al. In vivo visualization of glomerular microcirculation and hyperfiltration in streptozotocin-induced diabetic rats. Microcirculation 17, 103–112 (2010).
Xu, C. et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. http://dx.doi.org/10.1038/ki.2013.286.
Gil, N. et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes 61, 208–216 (2012).
Nieuwdorp, M. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55, 1127–1132 (2006).
Nieuwdorp, M. et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55, 480–486 (2006).
Broekhuizen, L. N. et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53, 2646–2655 (2010).
Deckert, T. et al. Size and charge selectivity of glomerular filtration in type 1 (insulin-dependent) diabetic patients with and without albuminuria. Diabetologia 36, 244–251 (1993).
Lemley, K. V. et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J. Am. Soc. Nephrol. 11, 2095–2105 (2000).
Schmidt, E. P. et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 (2012).
Zuurbier, C. J., Demirci, C., Koeman, A., Vink, H. & Ince, C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J. Appl. Physiol. 99, 1471–1476 (2005).
Rubio-Gayosso, I., Platts, S. H. & Duling, B. R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 290, H2247–H2256 (2006).
Nieuwdorp, M. et al. Tumor necrosis factor-α inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 202, 296–303 (2009).
Henry, C. B. & Duling, B. R. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 279, H2815–H2823 (2000).
Tumova, S., Woods, A. & Couchman, J. R. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32, 269–288 (2000).
Multhaupt, H. A. & Couchman, J. R. Heparan sulfate biosynthesis: methods for investigation of the heparanosome. J. Histochem. Cytochem. 60, 908–915 (2012).
Schwartz, N. Biosynthesis and regulation of expression of proteoglycans. Front. Biosci. 5, D649–D655 (2000).
Bishop, J. R., Schuksz, M. & Esko, J. D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 (2007).
Salmon, A. H. et al. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc. Res. 83, 24–33 (2009).
Satchell, S. C., Anderson, K. L., Tooke, J. E., Bates, D. O. & Mathieson, P. W. Angiopoietin 1 increases the integrity of human glomerular endothelial cell monolayers. J. Am. Soc. Nephrol. 13, 496A (2002).
Foster, R. R. et al. VEGF-C promotes survival in podocytes. Am. J. Physiol. Renal Physiol. 291, F196–F207 (2006).
Foster, R. R. et al. Vascular endothelial growth factor-C, a potential paracrine regulator of glomerular permeability, increases glomerular endothelial cell monolayer integrity and intracellular calcium. Am. J. Pathol. 173, 938–948 (2008).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Sison, K. et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J. Am. Soc. Nephrol. 21, 1691–1701 (2010).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Traub, O. & Berk, B. C. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 18, 677–685 (1998).
Segal, S. S. Regulation of blood flow in the microcirculation. Microcirculation 12, 33–45 (2005).
Forbes, M. S., Thornhill, B. A., Park, M. H. & Chevalier, R. L. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am. J. Pathol. 170, 87–99 (2007).
Zhao, H. J. et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 17, 2664–2669 (2006).
Nakagawa, T. et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 18, 539–550 (2007).
Kanetsuna, Y. et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am. J. Pathol. 170, 1473–1484 (2007).
Kidokoro, K. et al. Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J. Am. Soc. Nephrol. 24, 1139–1150 (2013).
Furusu, A. et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney Int. 53, 1760–1768 (1998).
Heeringa, P. et al. Renal expression of endothelial and inducible nitric oxide synthase, and formation of peroxynitrite-modified proteins and reactive oxygen species in Wegener's granulomatosis. J. Pathol. 193, 224–232 (2001).
Heeringa, P., Steenbergen, E. & van Goor, H. A protective role for endothelial nitric oxide synthase in glomerulonephritis. Kidney Int. 61, 822–825 (2002).
Zanchi, A. et al. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 57, 405–413 (2000).
Slater, S. C. et al. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int. J. Biochem. Cell Biol. 44, 1482–1490 (2012).
Tarbell, J. M. & Ebong, E. E. The endothelial glycocalyx: a mechano-sensor and transducer. Sci. Signal. 1, pt8 (2008).
Williams, M. E. Diabetic nephropathy: the proteinuria hypothesis. Am. J. Nephrol. 25, 77–94 (2005).
Morigi, M. et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am. J. Pathol. 166, 1309–1320 (2005).
Akilesh, S. et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl Acad. Sci. USA 105, 967–972 (2008).
George, M. et al. Renal thrombotic microangiopathy in mice with combined deletion of endocytic recycling regulators EHD3 and EHD4. PLoS ONE 6, e17838 (2011).
Hillege, H. L. et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106, 1777–1782 (2002).
Matsushita, K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375, 2073–2081 (2010).
Chappell, D. et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 107, 776–784 (2007).
Becker, B. F., Chappell, D., Bruegger, D., Annecke, T. & Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc. Res. 87, 300–310 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol 9, 717–725 (2013). https://doi.org/10.1038/nrneph.2013.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.197
This article is cited by
-
Elevated urine albumin creatinine ratio increases cardiovascular mortality in coronary artery disease patients with or without type 2 diabetes mellitus: a multicenter retrospective study
Cardiovascular Diabetology (2023)
-
Positive association between circulating Caveolin-1 and microalbuminuria in overt diabetes mellitus in pregnancy
Journal of Endocrinological Investigation (2023)
-
Immunomodulation by endothelial cells — partnering up with the immune system?
Nature Reviews Immunology (2022)
-
Association between depression symptoms and moderately increased levels of the inflammation marker albuminuria is explained by age and comorbidity
Scientific Reports (2022)
-
Endothelial glycocalyx is damaged in diabetic cardiomyopathy: angiopoietin 1 restores glycocalyx and improves diastolic function in mice
Diabetologia (2022)