Abstract
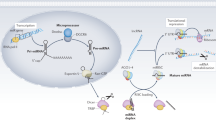
Chronic kidney disease (CKD) is characterized by tubulointerstitial deposition of extracellular matrix, tubular atrophy and dilatation; the replacement of organ architecture by connective tissue results in progressive loss of organ function. Micro (mi)RNAs are important mediators of tissue fibrosis under various pathological conditions and are of potential therapeutic relevance. These short, noncoding nucleotides (∼22 bases) regulate target messenger RNAs at the post-transcriptional level. Several hundred miRNAs regulate a considerable amount of the human genome and are involved in virtually all biological processes, including cellular proliferation, apoptosis and differentiation. Thus, miRNA deregulation often results in impaired cellular function and development of disease. Here, we summarize the current knowledge on the role of miRNAs in CKD, with particular emphasis on hypertensive kidney disease, diabetic nephropathy, glomerular biology, and IgA nephropathy. Identification of miRNA regulation and function in renal pathology may pinpoint miRNAs as new therapeutic targets in kidney fibrosis and related diseases. A new class of RNA therapeutics, that is, miRNA modulators (such as antagomirs) have been developed, which enable specific targeting of miRNAs and respective downstream gene networks in vivo, thus influencing the mechanisms that underlie disease initiation or progression. The therapeutic potential of miRNA-based treatment strategies in CKD are discussed.
Key Points
-
Chronic kidney diseases are associated with the development of tubulointerstitial fibrosis, which leads to progressive loss of organ function
-
Micro (mi)RNAs are endogenous, single-stranded short RNA molecules that regulate approximately 50% of the genome
-
miRNAs are deregulated in kidney diseases and influence the development of kidney fibrosis and dysfunction
-
Development of miRNA-based therapeutic strategies may attenuate or even reverse kidney fibrosis and dysfunction
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Eddy, A. A. Molecular basis of renal fibrosis. Pediatr. Nephrol. 15, 290–301 (2000).
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 (2010).
Foley, R. N., Parfrey, P. S. & Sarnak, M. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32, 112–119 (1998).
Kumar, V., Abbas, A. K. & Fausto, N. Pathologic Basis of Disease 87–118 (Elsevier Saunders, Philadelphia, 2005).
Quan, T. E., Cowper, S. E. & Bucala, R. The role of circulating fibrocytes in fibrosis. Curr. Rheumatol. Rep. 8, 145–150 (2006).
Willis, B. C., du Bois, R. M. & Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 3, 377–382 (2006).
Kalluri, R. & Neilson, E. G. Epithelial–mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784 (2003).
Bucala, R., Spiegel, L. A., Chesney, J., Hogan, M. & Cerami, A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1, 71–81 (1994).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Zeisberg, M. & Duffield, J. S. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 21, 1247–1253 (2010).
van Rooij, E. et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105, 13027–13032 (2008).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008).
Liu, G. et al. MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Thum, T. et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116, 258–267 (2007).
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
MicroCosm Targets, Version 5. EMBL–EBI database [online], (2010).
Chiang, H. R. et al. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev. 24, 992–1009 (2010).
Cai, X., Hagedorn, C. H. & Cullen, B. R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10, 1957–1966 (2004).
Lee, Y. et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 (2004).
Lee, Y. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 (2003).
Gregory, R. I. et al. The microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Valencia-Sanchez, M. A., Liu, J., Hannon, G. J. & Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20, 515–524 (2006).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Guo, H., Ingolia, N. T., Weissman, J. S. & Bartel, D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Harvey, J. M. et al. Renal biopsy findings in hypertensive patients with proteinuria. Lancet 340, 1435–1356 (1992).
Zucchelli, P. & Zuccalà, A. Primary hypertension—how does it cause renal failure? Nephrol. Dial. Transplant. 9, 223–225 (1994).
Marcantoni, C., Ma, L. J., Federspiel, C. & Fogo, A. B. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 62, 172–180 (2002).
Kincaid-Smith, P. Hypothesis: obesity and the insulin resistance syndrome play a major role in end-stage renal failure attributed to hypertension and labelled 'hypertensive nephrosclerosis'. J. Hypertens. 22, 1051–1055 (2004).
Sequeira López, M. L., Pentz, E. S., Nomasa, T., Smithies, O. & Gomez, R. A. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev. Cell. 6, 719–728 (2004).
Sequeira-López, M. L. et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J. Am. Soc. Nephrol. 21, 460–467 (2010).
Wang, G. et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am. J. Hypertens. 23, 78–84 (2010).
Tian, Z., Greene, A. S., Pietrusz, J. L., Matus, I. R. & Liang, M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res. 18, 404–411. (2008).
Sun, Y. et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 32, e188 (2004).
Bracken, C. P. et al. A double-negative feedback loop between ZEB1–SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 68, 7846–7854 (2008).
Paterson, E. L. et al. The microRNA-200 family regulates epithelial to mesenchymal transition. ScientificWorldJournal 8, 901–904 (2008).
Liu, Y. et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55, 974–982 (2010).
Fiedler, J., Gupta, S. & Thum, T. Micro-RNA based therapeutic approaches in the cardiovascular system. Cardiovasc. Ther. doi:10.1111/j.1755–5922.2010.00220.x.
Fioretto, P., Steffes, M. W., Brown, D. M. & Mauer, S. M. An overview of renal pathology in insulin-dependent diabetes mellitus in relationship to altered glomerular hemodynamics. Am. J. Kidney Dis. 20, 549–558 (1992).
Adler, S. Diabetic nephropathy: linking histology, cell biology, and genetics. Kidney Int. 66, 2095–2106 (2004).
Susztak, K., Raff, A. C., Schiffer, M. & Bottinger, E. P. Glucose induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006).
Krupa, A. et al. Loss of microRNA-192 promotes fibrogenesis in diabetic nephropathy. J. Am. Soc. Nephrol. 21, 438–447 (2010).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Comijn, J. et al. The two-handed E-box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7, 1267–1278 (2001).
Kato, M. et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc. Natl Acad. Sci. USA 104, 3432–3427 (2007).
Kato, M. et al. TGF-β activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat. Cell. Biol. 11, 881–889 (2009).
Jiang, B. H. & Liu, L. Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 102, 19–65 (2009).
Suzuki, A., Nakano, T., Mak, T. W. & Sasaki, T. Portrait of PTEN: messages from mutant mice. Cancer Sci. 99, 209–213 (2008).
Chung, A. C., Huang, X. R., Meng, X. & Lan, H. Y. miR-192 mediates TGF-β/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol. 21, 1317–1325 (2010).
Wang, B. et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of TGFβ. Diabetes 59, 1794–1802 (2010).
Du, B. et al. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 584, 811–816 (2010).
Wang, Q. et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 22, 4126–4135 (2008).
Fleissner, F. et al. Asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ. Res. 107, 138–143 (2010).
Shi, S. et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J. Am. Soc. Nephrol. 19, 2159–2169 (2008).
Wang, G. et al. Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab. Invest. 90, 98–103 (2010).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Lanford, R. E. et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 (2010).
Patrick, D. M. et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J. Clin. Invest. 120, 3912–3916 (2010).
Thum, T. et al. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J. Clin. Invest. 121, 461–462 (2011).
Kim, V. N. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell. Biol. 6, 376–385 (2005).
Ebert, M. S. et al. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 4, 721–726 (2007).
Xiao, J. et al. Novel approaches for gene-specific interference via manipulating actions of microRNAs: examination on the pacemaker channel genes HCN2 and HCN4. J. Cell. Physiol. 212, 285–292 (2007).
Sayed, D. et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol. Biol. Cell. 19, 3272–3282 (2008).
Krützfeldt, J. et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucl. Acids Res. 35, 2885–2892 (2007).
Martinez, J., Patkaniowska, A., Urlaub, H., Luhrmann, R. & Tuschl, T. Singlestranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110, 563–574 (2002).
Jazbutyte, V. & Thum, T. MicroRNA-21: from cancer to cardiovascular disease. Curr. Drug Targets 11, 926–935 (2010).
Author information
Authors and Affiliations
Contributions
J. M. Lorenzen and T. Thum made equal contributions to researching data for the article and to writing the article. H. Haller provided substantial discussion of the content. J. M. Lorenzen, H. Haller and T. Thum contributed equally to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lorenzen, J., Haller, H. & Thum, T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7, 286–294 (2011). https://doi.org/10.1038/nrneph.2011.26
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.26
This article is cited by
-
MiR-144-3p inhibits the proliferation and metastasis of lung cancer A549 cells via targeting HGF
Journal of Cardiothoracic Surgery (2022)
-
microRNA-338-3p suppresses lipopolysaccharide-induced inflammatory response in HK-2 cells
BMC Molecular and Cell Biology (2022)
-
SIRT7 silencing by miR-152-3p confers cell apoptosis and renal functional impairment induced by renal ischaemia/reperfusion injury
International Urology and Nephrology (2022)
-
Prevalence of miR146a Gene Polymorphisms in Diabetic and Non-diabetic Patients with Chronic Kidney Disease
Iranian Journal of Science and Technology, Transactions A: Science (2022)
-
Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling
Molecular Medicine (2021)