Key Points
-
Coeliac disease is a T cell-mediated enteropathy that has an autoimmune component and is induced by dietary wheat gluten. The ability to access to the targeted tissue during active (from patients on a gluten-containing diet) and non-active (from patients on a gluten-free diet) disease conditions makes it a unique human model to obtain insights into autoimmune disorders.
-
Coeliac disease is a multigenic complex immune disorder. The main genetic factors associated with coeliac disease are the MHC class II genes that encode HLA-DQ2 and HLA-DQ8. They contribute to at least 30% of the genetic heritability of the disease; the non-HLA genes identified to date contribute to only 3–4%.
-
The tissue enzyme transglutaminase 2 requires inflammatory signals to become activated in the tissue environment. Once it is activated, it deamidates gluten peptides, introducing negative charges that increase the binding affinity of the gluten peptides for HLA-DQ2 and HLA-DQ8.
-
Resistance to proteolytic cleavage and post-translational modifications of gluten, combined with particular physicochemical properties of HLA-DQ2 and HLA-DQ8 molecules, provide the basis for the association of coeliac disease with HLA-DQ2 or HLA-DQ8.
-
The presence of inflammatory mediators in the tissue environment may explain why intestinal dendritic cells induce an inflammatory response instead of a regulatory gluten-specific immune response; their presence may also explain why effector T cells become resistant to the inhibitory effects of regulatory T cells.
-
Intraepithelial cytotoxic lymphocytes require signals from target tissue cells to become licensed killer cells and to mediate tissue damage. In particular, interleukin-15 and non-classical MHC molecules expressed by intestinal epithelial cells reduce the activation threshold and promote the lytic activity of cytotoxic T cells by upregulating and activating natural killer cell receptors.
Abstract
Coeliac disease is an inflammatory disorder with autoimmune features that is characterized by destruction of the intestinal epithelium and remodelling of the intestinal mucosa following the ingestion of dietary gluten. A common feature of coeliac disease and many organ-specific autoimmune diseases is a central role for T cells in causing tissue destruction. In this Review, we discuss the emerging hypothesis that, in coeliac disease, intestinal tissue inflammation — induced either by infectious agents or by gluten — is crucial for activating T cells and eliciting their tissue-destructive effector functions.
Similar content being viewed by others
Main
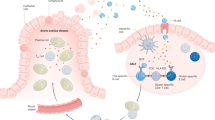
Coeliac disease is a gluten-sensitive enteropathy that develops in genetically susceptible individuals following exposure to dietary wheat gluten and similar proteins of barley and rye1. Although other environmental factors might be involved in the development or pathogenesis of coeliac disease, the observation that individuals with coeliac disease experience disease remission if they adhere to a gluten-free diet suggests that gluten has a key role in the pathogenesis of this disease. Susceptibility to coeliac disease is strongly associated with the MHC class II molecules HLA-DQ2 and HLA-DQ8, and the immune response directed against specific gluten antigens leads to the destruction of intestinal epithelial cells (IECs). Histological analysis has shown that coeliac disease is characterized by the loss of villi, crypt hyperplasia and lymphocytic infiltration that occurs mainly in the proximal part of the small bowel (duodenum and jejunum). Patients with coeliac disease typically also develop autoantibodies specific for the endogenous enzyme transglutaminase 2 (TG2; also known as TGM2) and antibodies to gluten. So, despite the fact that gluten (and not a self antigen) is the single causative agent, coeliac disease can be viewed as an organ-specific autoimmune disease (Box 1).
In general, the immune system is regarded as the main factor that is responsible for the pathogenesis of organ-specific autoimmunity, and the role of the targeted tissue in the initiation and progression of pathogenic T cell responses has been less well appreciated. Normal tissue function and homeostasis is maintained through balancing the need to activate an inflammatory immune response to eliminate invading pathogens with the need to minimize collateral damage to the tissue as a result of an over-exuberant immune response. There are numerous mechanisms involving the tissue environment that help to avoid the generation of inflammatory T cells and prevent those that are generated from causing tissue damage. One of the key checks takes place during T cell priming in lymphoid organs and determines whether a T cell becomes activated or deleted2,3 and, if activated, what type of effector or regulatory functions it will gain4. Dendritic cells (DCs) are central to this decision-making process and their regulatory properties are influenced by the signals they receive from the tissue environment. This has been well established for intestinal DCs, which gain immune regulatory properties from the gut environment and imprint gut-homing specificity on T cells5,6,7. Another crucial check takes place in the tissue and depends on cytotoxic T lymphocytes (CTLs) receiving the necessary additional signals to become fully functional effector cells8 (a process referred to as licensing) or become resistant to the inhibitory effects of regulatory T (TReg) cells9,10.
So why do T cells in coeliac diseases and organ-specific autoimmune diseases acquire an inflammatory phenotype and proliferate, instead of being deleted or gaining regulatory functions? How do they become 'licensed' to contribute to tissue destruction? In this Review, we use coeliac disease as a model to explore how components of the targeted tissue contribute to immune dysregulation and tissue damage. Studies of coeliac disease are facilitated by the easy access to the targeted tissue, the availability of samples from patients who are exposed (on a normal diet) or unexposed (on a gluten-free diet) to the inciting agent and a set of known genetic risk factors. Common factors to coeliac disease and organ-specific autoimmune diseases (Box 1) and the availability of extensive information in humans make coeliac disease a useful model for understanding the pathogenesis of organ-specific autoimmunity in humans.
Insights from genetics
The main genetic factors associated with coeliac disease are the MHC class II genes that encode HLA-DQ2 and HLA-DQ8; almost all patients with coeliac disease express one of these HLA molecules11,12. However, HLA-DQ2 and HLA-DQ8 are also common among healthy individuals, which suggests that additional genetic and/or environmental factors are involved. A large discrepancy in concordance rates between monozygotic twins and HLA-identical dizygotic twins also suggests the involvement of non-HLA genes13. Recent genome-wide association studies have led to the identification of several non-HLA risk loci for coeliac disease14,15 (Table 1). These risk loci are linkage disequilibrium blocks that contain a limited number of genes. In a few instances the susceptibility genes and the causative mutations have been identified. The individual effects of the non-HLA coeliac disease genes seem to be modest, and the collective effect of the associated genes described so far has been estimated to contribute to only 3–4% of the disease genetic heritability. The contribution of the HLA loci to disease heritability is estimated to be 30% or greater14. Future studies are expected to uncover more genetic regions that contribute to the risk of developing coeliac disease, possibly revealing the involvement of several hundred genes, which would be consistent with the fact that coeliac disease is a heterogeneous disorder.
Much work remains to be done to identify the causative mutations and to understand how they are involved in disease pathogenesis. Most of the candidate genes encode immunologically relevant proteins that affect the function of antigen-presenting cells (APCs) or T cells. Of particular interest is the locus on chromosome 4q27 that contains the genes encoding interleukin-2 (IL-2) and IL-21, among other genes. IL-21 is important for the proliferation and function of T cells and natural killer (NK) cells and for the differentiation of B cells into memory B cells and plasma cells16. Another interesting region is the locus on chromosome 2q12 that contains IL-18 receptor 1 (IL18R1) and IL-18 receptor accessory protein (IL18RAP), which encode the α- and β-chains of the IL-18 receptor, respectively. Mature IL-18, which is expressed in the intestinal mucosa of patients with coeliac disease but not in healthy controls17, induces T cells to synthesize the pro-inflammatory cytokine interferon-γ (IFNγ). Another risk locus that may also be involved in the genetic control of IFNγ production in coeliac disease is on chromosome 3q25 and contains IL12A, which encodes the p35 subunit of the p35–p40 heterodimeric IL-12 cytokine. IL-12 is produced by APCs and promotes T helper 1 cell differentiation and IFNγ secretion. Despite the finding that IFNγ is produced at high levels in coeliac lesions, mRNA encoding IL-12p40 is not present in coeliac lesions18, suggesting that IL12A expression might instead be implicated in T cell priming in the mesenteric lymph nodes.
Interestingly, many of the loci associated with coeliac disease susceptibility are also implicated in several other autoimmune diseases. In particular, coeliac disease and type 1 diabetes share several susceptibility genes19 (Table 1), suggesting that the pathogenesis of these diseases involves common immune pathways. Some loci have distinct effects in the two diseases; for example, the candidate genes IL12A and LPP (LIM domain containing preferred translocation partner in lipoma) seem to predispose only to coeliac disease and not to type 1 diabetes19. Notably, the genetic studies carried out so far do not support the notion that intestinal barrier defects or enterocyte dysfunction have a genetic basis in coeliac disease. The increased permeability and enterocyte turnover observed in patients with active coeliac disease are therefore likely to be secondary to the intestinal inflammation.
The multigenic and stochastic nature of T cell-mediated organ-specific autoimmune diseases such as type 1 diabetes and coeliac disease supports the notion that these diseases are complex, requiring multiple 'hits' — including genetic and environmental factors — to develop. These diseases differ from monogenic inflammatory and autoimmune diseases20,21, in which a defect in a single gene is sufficient to induce the disease.
Gluten as an immune response trigger
Coeliac disease is triggered by exposure to gluten proteins in the diet. Accordingly, the disease can be controlled if patients refrain from eating gluten, whereas the disease recurs following oral gluten challenge, which has previously been used as a routine diagnostic test. Two features of gluten proteins might explain their ability to trigger immune responses. First, the high content of proline in gluten proteins makes them resistant to degradation by intestinal proteases such that they can be found as long fragments (10–50 residues) in the gut lumen22. Second, these gluten fragments are good substrates for the enzyme TG2, which converts certain glutamine residues to glutamate (Box 2). This process, known as deamidation, increases the ability of the gluten peptides to bind to HLA-DQ2 or HLA-DQ8 molecules23,24. Importantly, CD4+ T cells isolated from the intestinal lesions of patients with coeliac disease preferentially recognize gluten peptides deamidated by TG2 (Refs 25, 26). In addition, gluten can trigger CD8+ T cell responses in the lamina propria27 and may expand the intraepithelial lymphocyte population independently of MHC presentation28.
Although there is a clear role for gluten in activating gluten-specific T cells in the lamina propria, it remains unclear whether gluten also contains fragments that can stimulate cells of the innate immune system. In vitro studies of intestinal organ cultures, primary APCs, and epithelial and monocytic cell lines support the notion that gluten affects the innate immune system29,30,31,32,33,34,35,36. These studies have reported numerous and diverse effects of gluten on innate immune cells and other cells. The gluten-derived peptide most studied for its innate immune properties is the α-gliadin 31–43 peptide, which does not induce T cell-specific responses in the gut but has immune-stimulating effects29,30. The effects reported by different groups on innate or innate-like immune cells are diverse in terms of the response generated and the cells targeted. The effects on IECs include the induction of apoptosis29, upregulation of expression of the stress-inducible MHC class I polypeptide-related molecules (MICs)30 and inhibition of the epidermal growth factor receptor (EGFR) endocytic pathway, resulting in increased EGFR expression and activation31. A more general effect on non-T cells includes upregulation of the mitogen-activated protein (MAP) kinase p38, CD83 and IL-15 (Ref. 29).
In addition to the α-gliadin 31–43 peptide, various other gluten-derived peptides have been reported to have innate immune-stimulating properties (Fig. 1). One study described a gluten peptide with an amino acid sequence similar to that of MHC class I leader peptides, which bind to and increase cell surface expression of the non-classical MHC class I molecule HLA-E32. HLA-E is the ligand for the CD94–natural killer group 2, member A (NKG2A) and CD94–NKG2C NK cell receptors expressed by intraepithelial lymphocytes8. Other studies33,34 have described gluten peptides that can activate APCs. An involvement of Toll-like receptor 4 (TLR4) in gluten-mediated APC activation has been suggested by one group35. Finally, another set of gluten peptides was shown to activate CXC-chemokine receptor 3 (CXCR3) and myeloid differentiation primary response protein 88 (MYD88) in enterocytes, resulting in increased intestinal permeability36.
Diverse effects of gluten on intestinal epithelial cells (IECs) and antigen-presenting cells (APCs) have been described. The most commonly described gluten peptide that affects IECs is the α-gliadin 31–43 peptide (amino acid sequence: LGQQQPFPPQQPY). No specific receptor has been identified for this peptide; instead, it is thought to mediate its effects by altering cell trafficking and/or activating currently undefined cell stress pathways. This peptide has also been shown to activate mitogen-activated protein kinases (MAPKs) and upregulate the expression of interleukin-15 (IL-15), MHC class I polypeptide-related molecules (MICs) and epidermal growth factor receptor (EGFR). Other gluten peptides have also been reported to activate APCs by undefined mechanisms or by binding to Toll-like receptor 4 (TLR4). Finally, additional sets of gluten peptides were reported to stabilize the expression of the non-classical MHC class I molecule HLA-E, a ligand for the CD94 natural killer cell receptor family, or increase intestinal permeability following binding to CXC-chemokine receptor 3 (CXCR3). TNF, tumour necrosis factor.
It is currently unclear how gluten could have such a range of biological effects on innate immune cells and how it can bind to unrelated receptors; further study is therefore required to identify the molecular mechanisms involved. However, if confirmed, the possible role of gluten as an activator of the innate immune system might explain how inflammatory gluten-specific CD4+ T cell responses are induced and how CTLs become licensed to kill IECs. Based on the current data, we suggest that in genetically susceptible individuals gluten leads to the activation of cellular stress pathways or to the conversion of self molecules into ligands for immune receptors (such as TLRs), which in turn could trigger the release of pro-inflammatory mediators that promote the development of inflammatory T cell responses.
TG2 activation and gluten-specific T cell responses
The role of post-translational modifications of proteins in autoimmunity and coeliac disease is now well recognized37. It is thought that post-translational modifications can enhance the affinity of peptides for particular MHC molecules and/or create neoantigens that may promote pathogenic T cell responses. In the context of coeliac disease, post-translational modification of gluten by TG2 is thought to have a crucial role in the gluten-specific CD4+ T cell response25,26. Recent in vivo experiments in mice indicate that TG2 is inactive in the intestinal mucosa in the resting state but is activated following treatment of the animals with polyinosinic–polycytidylic acid (polyI:C; a ligand of TLR3)38. A possible involvement of TLR3 ligation in TG2 activation suggests that infection with certain viruses (for example, double-stranded RNA viruses such as rotavirus) might be involved in vivo through the provision of ligands for TLR3. Alternatively, it is also possible that inflammation-induced tissue destruction and the subsequent release of intracellular TG2 might trigger its activation. Indeed, the initial CD4+ T cell response may be directed against native gluten peptides and this may be sufficient to induce tissue inflammation and consequently TG2 activation. Support for this order of events has been provided by recent studies using an HLA-DQ8-transgenic mouse model39 and by the observation that T cell responses in children seem to be mainly directed against native gluten peptides40. Whether HLA-DQ2-restricted gluten-specific T cell responses can be initiated in the absence of TG2 activation remains to be determined.
Molecular basis for the HLA-DQ association
Understanding why particular MHC class II molecules predispose people to coeliac disease, and indeed why other MHC molecules predispose to other diseases, has been a subject of intense research. There is now good evidence that the MHC association in coeliac disease is linked to the preferential binding by HLA-DQ2 and HLA-DQ8 molecules to proteolysis-resistant gluten peptides that have negatively charged glutamate residues introduced by TG2 (Refs 22, 23, 24, 25, 26). Moreover, several studies show how the combination of an antigen, tissue-derived factors and particular MHC molecules can determine the amplitude of specific T cell responses and development of disease. Koning and colleagues41 proposed a quantitative model for coeliac disease development involving a threshold effect, in which the number of gluten peptide–MHC (HLA-DQ2 or HLA-DQ8) complexes expressed on the surface of APCs is a limiting factor that defines the magnitude of the gluten-specific CD4+ T cell response and the consequent induction of intestinal tissue damage. This model was based on the finding that susceptibility to coeliac disease is higher in individuals who are homozygous for HLA-DQ2 or HLA-DQ8 than in individuals who are heterozygous for these alleles, suggesting gene-dose effects of the HLA-DQ molecules42,43. For HLA-DQ2, gene dose was shown to be directly related to the magnitude and breadth of gluten-specific T cell responses41. Recent studies39,44,45 analysing other aspects of the HLA-DQ2- and HLA-DQ8-restricted CD4+ T cell response have provided additional support for and perspectives on the importance of threshold in the priming of gluten-specific T cell responses.
HLA-DQ8 and the gluten-specific CD4+ T cell response. Unlike some other HLA-DQ alleles, HLA-DQ8 does not have an aspartate residue at position 57 of the β-chain (Aspβ57). This polymorphism has been shown to be important in determining susceptibility to type 1 diabetes46 and may also be important in coeliac disease39. The lack of Aspβ57 in HLA-DQ8 creates a large, positively charged P9 pocket in the peptide-binding groove. This unique feature of the HLA-DQ8 P9 pocket bestows a preference for binding a negatively charged residue47,48. HLA-DQ8 Aspβ57 also seems to select T cell populations, responding to non-charged native (that is, not deamidated) peptides, that express T cell receptors (TCRs) with a negatively charged complementarity-determining region 3β (CDR3β); this may help to stabilize the weak interaction between the native gluten peptide and HLA-DQ8 (Ref. 39). These TCRs were shown to cross-react with the deamidated gluten peptide and can therefore also be involved in T cell responses after TG2 becomes activated. Once TG2 is activated, the T cell response becomes directed towards the deamidated version of the peptide, which binds with higher affinity to HLA-DQ8 and does not require TCRs with a negatively charged residue in the CDR3β loop. Consistent with this, immunization of HLA-DQ8-transgenic mice with a mixture of native and deamidated gluten peptides resulted in the recruitment of a broader TCR repertoire and a gluten-specific T cell response of higher magnitude than that induced by immunization with deamidated peptide alone39. In accordance with the mouse model, human gluten-specific HLA-DQ8-restricted T cells were found to express TCRs with a charged residue in CDR3β and to recognize both native and deamidated forms of gluten peptides. Together, these findings led us to propose a model (Fig. 2a) in which HLA-DQ8 association with coeliac disease can be explained by its ability to select distinct but cross reactive TCRs in response to native and deamidated peptides and thereby amplify the gluten-specific T cell response. Interestingly, T cells bearing a similar signature charge in CDR3β have also been described in the early β-islet infiltrate of non-obese diabetic (NOD) mice49, suggesting that similar mechanisms may apply to type 1 diabetes.
a | The initial gluten-specific T cell response in HLA-DQ8-positive patients with coeliac disease is directed against native (undeamidated) gluten peptides and involves T cell receptors (TCRs) with negatively charged residues that interact with positively charged structures of HLA-DQ8, thereby stabilizing the peptide–MHC–TCR interaction. Half of the T cells that respond to the native gluten peptide also recognize (in some cases with higher avidity) the deamidated form of the gluten peptide that is subsequently generated by transglutaminase 2 (TG2; also known TGM2). T cell responses to deamidated gluten peptides, which bind better to HLA-DQ8, do not depend on TCRs with negatively charged residues and so have a distinct and diverse TCR repertoire. The combined T cell response is greater than the response to the native or deamidated peptides alone and this helps to reach the threshold needed for priming a pathological T cell response. b | Gluten-derived T cell epitopes are rich in proline residues (making them resistant to gastrointestinal proteolysis) and harbour glutamate residues that are introduced by TG2-mediated deamidation. HLA-DQ2 has a preference for binding negatively charged residues (such as glutamate) in P4, P6 and P7 pockets, whereas HLA-DQ8 has a preference for binding negatively charged residues in P1 and P9. Furthermore, HLA-DQ2 preferably binds peptides with proline at position P1, and such peptides may be present in high numbers owing to their protease resistance. This would support the presentation of higher levels of peptide–HLA-DQ2 complexes than peptide–HLA-DQ8 complexes, increasing the likelihood of reaching a threshold needed for the priming of a pathogenic T cell response. c | There are two types of HLA-DQ2 molecule: HLA-DQ2.5 (encoded by HLA-DQA1*05 and HLA-DQB1*02) and HLA-DQ2.2 (encoded by HLA-DQA1*0201 and HLA-DQB1*02). Only HLA-DQ2.5 is a strong risk factor for coeliac disease. Recent work shows that HLA-DQ2.5 binds gluten peptides with higher kinetic stability and allows longer gluten presentation than HLA-DQ2.2, thereby increasing the likelihood of reaching the threshold required for successful priming of pathogenic T cells. APC, antigen-presenting cell.
HLA-DQ2 and the gluten-specific CD4+ T cell response. Whereas HLA-DQ8 has a preference for binding negatively charged residues in the P1 and P9 pockets47,48, HLA-DQ2 has a preference for binding negatively charged residues in the P4, P6 and P7 pockets50 (Fig. 2). These distinct HLA-DQ2 and HLA-DQ8 binding signatures result in the selection of T cell responses to different α-gliadin epitopes44,49. HLA-DQ2 seems to have a unique ability to accommodate proline residues in the P1 pocket, and notably most of the characterized HLA-DQ2-restricted epitopes that are recognized by gluten-specific T cells have a proline residue at P1 (Ref. 51). These epitopes probably cannot bind to HLA-DQ8. The fact that HLA-DQ2 is better suited than HLA-DQ8 to bind the proline-rich gluten peptides that survive gastrointestinal digestion may be the reason why HLA-DQ2 is a stronger susceptibility determinant for coeliac disease than HLA-DQ8 (Fig. 2b).
Two variants of HLA-DQ2 molecules exist: HLA-DQ2.5 and HLA-DQ2.2. HLA-DQ2.5, which is encoded by the HLA-DQA1*05 (α-chain) and HLA-DQB1*02 (β-chain) alleles, is associated with coeliac disease and many other autoimmune diseases51,52. By contrast, HLA-DQ2.2, which is encoded by the HLA-DQA1*0201 and HLA-DQB1*02 alleles, has, on its own, a very low risk for coeliac disease and is not associated with other autoimmune diseases. However, HLA-DQ2.2-positive individuals who are HLA-DR5DQ7/HLA-DR7DQ2.2 heterozygous do have a high risk of developing coeliac disease11. These individuals express the HLA-DQ2.5 molecule, but the encoding alleles (HLA-DQA1*05 and HLA-DQB1*02) are located on opposite chromosomes. It was therefore unclear, until recently, why HLA-DQ2.2 is not a significant risk factor for coeliac disease, given that HLA-DQ2.5 and HLA-DQ2.2 have very similar peptide binding motifs53 and that T cells from HLA-DQ2.5 individuals can recognize many gluten epitopes presented by HLA-DQ2.2 (Ref. 51). A recent study has provided insight into this issue by showing that HLA-DQ2.5 binds gluten peptides with a higher affinity than HLA-DQ2.2 and supports prolonged gluten presentation by APCs45 (Fig. 2c). The functional difference between the two molecules was attributed to a polymorphism in the HLA-DQ α-chain, which in HLA-DQ2.5 is a tyrosine residue but in HLA-DQ2.2 is a phenylalanine residue; only the tyrosine residue can form a hydrogen bond to the gluten peptide main chain, facilitating prolonged peptide–MHC association.
The importance of peptide–MHC half-life for the in vivo priming of CD8+ T cells was recently shown using an MHC class I-transgenic mouse model54. Substitution of a single residue in an MHC class I peptide ligand, which did not affect TCR recognition but which gave a moderate difference in peptide–MHC half-life (6 versus 2.3 hours), resulted in an enormous difference (30,000-fold) in the in vivo antigenicity of the peptides. The authors showed that the degree of CD8+ T cell activation by DCs in the draining lymph node was determined by a threshold of cognate peptide–MHC complexes54. The peptide binding off-rate was found to be the key determinant in this process. So, the better ability of HLA-DQ2.5 to remain loaded with its peptide cargo, compared with HLA-DQ2.2, should facilitate DC priming of naive T cells in the secondary lymphoid tissue. These findings could explain the difference between these two HLA-DQ2 variants in the susceptibility they confer to coeliac disease and possibly more generally to autoimmune diseases.
Together, these studies reveal how, in combination, a glutamine- and proline-rich peptide, deamidation of the peptide by intestinal TG2 and the particular physicochemical properties of HLA-DQ2.5 and HLA-DQ8 lead to the induction of a gluten-specific CD4+ T cell response of sufficient amplitude to promote intestinal damage (the threshold effect) (Fig. 2).
Effect of inflammation on gut homeostasis
Effect of tissue-derived inflammatory factors on oral tolerance. The largest part of the mammalian immune system is associated with the gastrointestinal tract, where it is faced with the formidable task of mounting an effective response to pathogenic microorganisms while remaining unresponsive to innocuous food antigens and commensal bacteria. The default response by the intestinal immune system to orally administered protein is the induction of forkhead box P3 (FOXP3)+ TReg cells6,7 that secrete anti-inflammatory cytokines (such as transforming growth factor-β (TGFβ), IL-10 and IL-4) and promote the production of IgA antibodies55 — an active process referred to as oral tolerance56. These TReg cells are induced by intestinal DCs that are modified by enterocyte-derived factors, such as retinoic acid and TGFβ, that confer tolerogenic properties on the DCs57,58. Oral tolerance has been extensively studied in rodents59 and has also been shown to occur in humans60.
However, unlike in healthy individuals, inflammatory CD4+ T cell responses to dietary gluten in the small intestinal mucosa can be observed in patients with coeliac disease61. One possible explanation for this observation is that there is an alteration to the intestinal environment that affects the differentiation and/or function of FOXP3+ TReg cells in individuals with coeliac disease. The intestinal mucosa of patients with coeliac disease is characterized by the presence of high levels of the pro-inflammatory cytokines IL-15 (produced in the epithelium62,63 and lamina propria64) and IFNα65,66,67. The cellular source of IFNα in the coeliac lesions is not clear, as many cells can produce IFNα. Plasmacytoid DCs are known to produce large amounts of IFNα and were observed in high numbers in coeliac lesions in one study68 but not in another study69. Some observations suggest an intriguing potential link between high levels of IL-15 and IFNα in the intestinal mucosa and loss of oral tolerance. For example, IFNα treatment (for chronic hepatitis)70 and rotavirus infections71 were found to precipitate the induction of inflammatory anti-gluten responses and the generation of TG2-specific antibodies. Furthermore, our preliminary findings using HLA-DQ8-transgenic mice suggest that IL-15 alters the phenotype and function of intestinal DCs and prevents the induction of FOXP3+ TReg cells after oral challenge with gluten (B.J., unpublished observations). Based on these observations, we propose that intestinal DCs stimulated by pro-inflammatory mediators such as IL-15 and IFNα may lose their tolerogenic phenotype and instead promote the differentiation of pro-inflammatory T cells (despite the continued presence of tolerogenic mediators such as retinoic acid and TGFβ) (Fig. 3).
a | The default response to oral antigens is the induction of regulatory T (TReg) cells that produce transforming growth factor-β (TGFβ) and interleukin-10 (IL-10). The existence of gluten-specific TReg cells in humans is yet to be demonstrated. b | Under inflammatory conditions, the expression of pro-inflammatory mediators is upregulated in the intestinal environment and dendritic cells (DCs) may acquire the ability to promote the differentiation of T cells that produce pro-inflammatory cytokines such as interferon-γ (IFNγ) and possibly IL-21. This may involve the production of IL-12 and possibly also IFNα by DCs in the mesenteric lymph node. Future studies are needed to determine whether acquisition of an inflammatory phenotype by DCs in patients with coeliac disease results directly following exposure to gluten or is secondary to an infection (for example with rotavirus) and the presence of pro-inflammatory mediators such as IL-15 and IFNα in the intestinal environment. RA, retinoic acid; TH1, T helper 1.
Effect of tissue-derived inflammatory factors on T cell regulation. Several studies have analysed whether effector CD4+ and CD8+ T cells become resistant to immune regulation in the presence of IL-15 (Refs 9, 10). For example, in the joints of patients with juvenile idiopathic arthritis, IL-15 was found in the synovial fluid and, when added to in vitro cultures, it abrogated the suppressive activity of FOXP3+ TReg cells9. In addition, IL-15 was shown to prevent the inhibitory effects of TGFβ on intraepithelial lymphocytes72. These findings suggest that effector T cells in the gut mucosa of patients with coeliac disease, which contains high levels of IL-15 (Refs 64, 65, 66), might be insensitive to the regulatory effects of TGFβ and FOXP3+ T cells (Fig. 4). In addition, IL-21, which is also highly expressed in the mucosa of patients with coeliac disease73, similarly renders T cells resistant to the effects of FOXP3+ TReg cells74. Together, these findings may explain why, despite the presence of FOXP3+ TReg cells in autoimmune disease9, destructive effector T cell responses are not inhibited. Whether IFNα has similar effects remains to be determined.
a | In steady-state conditions, regulatory T (TReg) cells contribute to maintenance of homeostasis in the intestinal tissue by inhibiting the induction of gluten-specific T cell responses and by promoting the production of IgA. b | During inflammation or infection, gluten-specific CD4+ T helper 1 (TH1) cells secrete pro-inflammatory mediators such as interferon-γ (IFNγ) or interleukin-21 (IL-21), which act on intestinal epithelial cells (IECs), promote activation of intraepithelial cytotoxic T lymphocytes (CTLs) and block the inhibitory effects of forkhead box P3 (FOXP3)+ TReg cells. In addition, gluten-specific TH1 cells are thought to help B cells to produce gluten- and transglutaminase 2 (also known as TGM2)-specific IgG and IgA antibodies. These antibodies may contribute to inflammation by engaging Fc receptors (FcRs) on antigen-presenting cells (APCs) and may contribute to extraintestinal manifestations through the deposition of immune complexes in tissues such as the skin and brain. Inflammation is also associated with upregulation of expression of the IgA receptor CD71 (also known as transferrin receptor) on the luminal surface of IECs. CD71-mediated transcytosis could facilitate the entry of IgA–gluten complexes into the intestinal mucosa. CSR, class switch recombination; DC, dendritic cell; TGFβ, transforming growth factor-β.
Tissue inflammation and licensing of CTLs
The role of CD4+ T cells in the induction of intestinal damage. Several lines of evidence indicate an essential role of CD4+ T cells in coeliac disease pathogenesis. These include: the HLA locus being the single most important genetic risk factor; the detection of gluten-specific, HLA-DQ-restricted CD4+ T cells in the intestinal mucosa of patients with TG2-specific antibodies; and the correlation between removal of gluten, loss of the gluten-specific CD4+ T cell effector response and the recovery of intestinal villi. However, there is now evidence, both in humans and mice, that CD4+ T cells are not the effector cell type that mediates tissue damage in coeliac disease. Instead, CD4+ T cells help to set up the necessary inflammatory environment that allows intraepithelial CTLs to induce tissue damage (Fig. 4). This could be achieved through the secretion of pro-inflammatory cytokines such as IFNγ75 and IL-21 (Ref. 76), which may promote epithelial cell destruction by intraepithelial CTL activation (see below). In addition, it is probable that gluten-specific CD4+ T cells help B cells to differentiate into plasma cells that secrete gluten- and TG2-specific IgA and IgG antibodies77. These antibodies may contribute to intestinal damage by increasing the transcellular transport of gluten from the lumen to the lamina propria and thereby amplifying the gluten-specific CD4+ T cell response78. Furthermore, the formation of immune complexes can trigger activating Fc receptors on APCs, leading to the release of pro-inflammatory mediators79 (Fig. 4). Of note, in addition to gluten-specific CD4+ T cells, gluten-specific CD8+ T cells have been found in the lamina propria27, but their possible role in coeliac disease pathogenesis remains to be established.
The role of intraepithelial CTLs in destruction of IECs and villous atrophy. In intestinal diseases, such as graft-versus-host disease80 and autoimmune enteropathies81, in which CD4+ T cells are viewed as the main effector cell mediating tissue damage, crypt cells are the primary targets of the destruction process and intraepithelial CTLs expand only moderately and late in the disease process. By contrast, coeliac disease is characterized by the presence of intact, hyperproliferative crypts82, suggesting that surface IECs are the main targets of the destructive process. Furthermore, intraepithelial CTLs expand early in the disease process and correlate with the presence of villous atrophy83. Finally, whereas approaches aimed at activating CD4+ T cells failed to induce villous atrophy84,85, there are now several mouse models showing that activation of intraepithelial CTLs by IL-15 expressed on IECs results in the destruction of IECs and villous atrophy86,87. This suggests that tissue remodelling caused by CD4+ T cells alone cannot explain villous atrophy observed in coeliac disease, and that other mechanisms such as the destruction of IECs by intraepithelial CTLs need to be invoked.
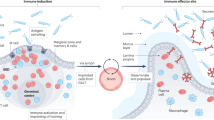
The role of intraepithelial CTLs in coeliac disease pathogenesis was initially disregarded because gluten-specific CD8+ T cells were not found and because HLA class I alleles do not seem to have a clear genetic effect on coeliac disease88. Independent of their antigen specificity, a role for intraepithelial CTLs as the key effector cell subset that mediates IEC destruction has been suggested based on the discovery that these cells in humans express the activating receptor NKG2D89. Expression of this receptor conferred potent co-stimulatory and direct cytotoxic functions on the CTLs, in particular following stimulation with IL-15 (Refs 89, 90).
In contrast to the equivalent mouse cells, human effector CD8+ T cells lack expression of the co-stimulatory receptor CD28 and instead express NK cell receptors that recognize non-classical MHC class I molecules and modulate TCR signalling64,89,91. In healthy individuals, intraepithelial CTLs express the inhibitory receptor CD94–NKG2A, the C-type lectin CD161 (also known as KLRB1) and low levels of the activating receptors NKG2D and CD94–NKG2C89,91,92. By contrast, intraepithelial CTLs from patients with coeliac disease lose their expression of the inhibitory receptor CD94–NKG2A92 and acquire high levels of expression of the activating receptors NKG2D90 and CD94–NKG2C92. At the same time, IECs upregulate the expression of ligands for these activating receptors: the MICs30,90 and HLA-E92, respectively.
In addition, IL-15, produced by IECs in patients with coeliac disease, has recently been shown to induce signalling in CTLs and can alter their function, in particular by upregulating the expression of NKG2D and co-stimulating the NKG2D cytotoxic signalling pathway89,90,93 (Fig. 5). Interestingly, NKG2D and IL-15 also induce the release of arachidonic acid93 and, potentially, leukotrienes, which are potent pro-inflammatory mediators94. Activation of intraepithelial CTLs by NKG2D and IL-15 could therefore not only contribute to the destruction of IECs but also promote nonspecific inflammation in the intestinal mucosa of patients with coeliac disease (Fig. 5). Interestingly, Vδ1+ intraepithelial γδ T cells are significantly increased in the intestinal epithelium of patients with coeliac disease95, suggesting that they may recognize stress-inducible ligands on IECs, such as MICs and CD1 (Refs 96, 97). However, in contrast to intraepithelial CTLs, intraepithelial γδ T cells remain present in the mucosa at high numbers for years after patients go on a gluten-free diet, implying that they do not have a role in the pathogenesis of the disease83. Instead, it has recently been suggested that they have a regulatory role, based on their ability to produce TGFβ, their expression of the inhibitory receptor CD94–NKG2A98 and on the observation that they are found in greater numbers in patients with latent coeliac disease with normal intestinal histology than in patients with active disease99.
Naive CD8+ T cells that are primed in the Peyer's patches or mesenteric lymph nodes undergo progressive maturation during their circulation in the lymph before populating the intestinal epithelium as effector T cells. Despite their effector T cell phenotype, intraepithelial cytotoxic T lymphocytes (CTLs) need to receive a second signal to gain effector functions. In a healthy intestinal environment, intraepithelial CTLs mainly express inhibitory CD94–natural killer group 2, member A (NKG2A) receptors, and intestinal epithelial cells (IECs) lack expression of the ligands HLA-E and MHC class I polypeptide-related molecules (MICs) for activating receptors, such as NKG2D. In coeliac disease, IECs express high levels of interleukin-15 (IL-15), together with MICs and HLA-E. IL-15 leads to the upregulation of NKG2D expression by intraepithelial CTLs and the cytotoxic signalling pathway associated with this receptor. Consequently, intraepithelial CTLs acquire killing activity and can target MIC-bearing IECs. Furthermore, intraepithelial CTLs of patients with coeliac disease express CD94–NKG2C–DAP12 immunoreceptor complexes that recognize HLA-E on IECs and can promote cytokine secretion, proliferation and cytotoxic activity of T cells in a T cell receptor (TCR)-independent manner. Activation of NKG2D in conjunction with IL-15 expression leads to the release of arachidonic acids (and possibly leukotrienes) by intraepithelial CTLs, which in turn can promote activation of intestinal granulocytes and inflammation. DC, dendritic cell; IFNγ, interferon-γ.
NK cell reprogramming of intraepithelial CTLs in coeliac disease and refractory sprue. The finding that NKG2D-mediated activation in intraepithelial CTLs could promote killing of IECs provided an explanation as to how intraepithelial CTLs might be involved in coeliac disease-associated tissue damage despite not being gluten specific. However, because NKG2D in humans does not associate with the immunoreceptor tyrosine-based activation motif (ITAM)-bearing adaptor molecule DAP12 (Ref. 100) and consequently fails to induce cytokine secretion and proliferation, it did not explain how these events could occur in patients with coeliac disease. The molecular basis for this paradox was clarified by the finding that a subset of intraepithelial CTLs in patients with coeliac disease underwent a profound genetic reprogramming of NK cell functions, becoming NK cell-like cells92. More specifically, the intraepithelial CTLs were shown to express DAP12 and activating NK cell receptor complexes, such as CD94–NKG2C, NKp44 and NKp46, that could mediate killing of NK cell targets, cytokine secretion and proliferation. Interestingly, acquisition of this NK cell-like phenotype was associated with a decrease in the level of transcripts encoding TCR α- and β-chains and a marked oligoclonal expansion92. Moreover, expression of HLA-E, the ligand for CD94–NKG2C, was found to be upregulated on IECs in active coeliac disease92.
This NK cell-like transformation of intraepithelial CTLs may be a crucial step that precedes refractory sprue and enteropathy-associated T cell lymphoma (EATL), which are rare but major complications of coeliac disease101 that are characterized by the presence of high numbers of intraepithelial CTLs with a NK cell-like phenotype and persistent villous atrophy despite a gluten-free diet102. An important role for IL-15 in refractory sprue and EATL has been shown by studies in transgenic mice103 and humans65. Transgenic mice expressing IL-15 under the control of an MHC class I promoter were shown to develop fatal CTL leukaemia with an NK cell-like CTL phenotype103. In vitro studies with human cells showed that IL-15 promoted the survival, expansion, cytotoxic activity and cytokine secretion of intraepithelial CTLs from patients with refractory sprue65. Whether chronic NKG2D activation participates in NK cell reprogramming and malignant transformation of CTLs remains to be established.
Overall, these studies suggest that expression of IL-15 and non-classical MHC class I molecules by stressed tissue cells and of activating NK cell receptors by effector T cells may determine the ability of T cells to induce tissue damage. In other words, expression of IL-15 and non-classical MHC class I molecules by tissue cells may licence effector T cells and trigger their killing activity. Although intraepithelial CTLs in coeliac disease seem not to be specific for gluten, they respond to the indirect effects of gluten that cause upregulation of IL-15 and non-classical MHC class I molecules on IECs. It remains to be determined whether high levels of IL-15 and non-classical MHC class I molecule expression by tissue cells is the consequence of cellular alterations related to mechanic or metabolic stress or is induced by infections. In that regard it is interesting that the expression of IL-15 and MICs is found on synoviocytes of patients with rheumatoid arthritis104, and that RAE1 (a mouse NKG2D ligand) is present on β-islet cells of NOD mice but not control mice105.
Unresolved issues and future directions
In this Review, we have described the recent advances in our understanding of the immunopathogenesis of coeliac disease, particularly in relation to the role of the MHC molecules HLA-DQ2 and HLA-DQ8 and mechanisms through which enterocytes are killed by intraepithelial CTLs. However, many issues remain unresolved. A set of new susceptibility loci (Table 1) have been discovered, but the identities of the causative mutations and how these mutations are involved in the coeliac disease pathogenesis remain to be defined. The mechanisms underlying the induction and loss of oral tolerance to gluten, how TG2-specific antibodies are generated, the role of immune complexes, the molecular mechanisms underlying the effects of gluten on innate immune cells and the role of IFNα are all issues that warrant further investigation. Furthermore, we still need to determine whether the greater prevalence of organ-specific autoimmune diseases such as type 1 diabetes or thyroiditis in patients with coeliac disease is the result not only of common susceptibility genes but also of immune activation pathways leading to systemic effects. In particular, immune complexes and high levels of IFNα may explain how the immune system may have an overall lower activation threshold. This hypothesis is supported by the finding that patients receiving IFNα for the treatment of hepatitis C virus infection have increased susceptibility to the development of coeliac disease70, type 1 diabetes106 and thyroiditis107. Finally, the presentation of coeliac disease seems to involve extraintestinal manifestations, for which the underlying mechanisms need to be defined. The development of mouse models, inspired by human studies, will allow the cause–effect relationships and new therapeutic avenues to be tested.
References
Green, P. H. & Cellier, C. Celiac disease. N. Engl. J. Med. 357, 1731–1743 (2007).
Janeway, C. A. Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11–16 (1992).
Matzinger, P. Friendly and dangerous signals: is the tissue in control? Nature Immunol. 8, 11–13 (2007).
Kalinski, P., Hilkens, C. M., Wierenga, E. A. & Kapsenberg, M. L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20, 561–567 (1999).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Meresse, B. & Jabri, B. NKG2 Receptor-Mediated Regulation Of Effector CTL Functions In The Human Tissue Microenvironment (eds Vivier, E. & Colonna, M.) (Springer, 2006).
Ruprecht, C. R. et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 201, 1793–1803 (2005).
Benahmed, M. et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J. Immunol. 182, 6763–6770 (2009).
Sollid, L. M. et al. Evidence for a primary association of celiac disease to a particular HLA-DQ α/β heterodimer. J. Exp. Med. 169, 345–350 (1989).
Spurkland, A., Sollid, L. M., Polanco, I., Vartdal, F. & Thorsby, E. HLA-DR and -DQ genotypes of celiac disease patients serologically typed to be non-DR3 or non-DR5/7. Hum. Immunol. 35, 188–192 (1992).
Nistico, L. et al. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut 55, 803–808 (2006).
van Heel, D. A. et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nature Genet. 39, 827–829 (2007). The first genome wide association study of coeliac disease.
Hunt, K. A. et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nature Genet. 40, 395–402 (2008).
Leonard, W. J. & Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol. 5, 688–698 (2005).
Salvati, V. M. et al. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut 50, 186–190 (2002).
Nilsen, E. M. et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115, 551–563 (1998).
Smyth, D. J. et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N. Engl. J. Med. 359, 2767–2777 (2008).
Martinon, F. & Tschopp, J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117, 561–574 (2004).
Wildin, R. S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature Genet. 27, 18–20 (2001).
Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279 (2002). This paper establishes a relationship between the inability of intestinal proteases to break down gluten and the generation of an immunodominant gluten peptide for HLA-DQ2-positive patients. It also introduces bacterial prolyl endopeptidase as a potential supplement therapy in coeliac disease.
Arentz-Hansen, H. et al. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191, 603–612 (2000).
Moustakas, A. K. et al. Structure of celiac disease-associated HLA-DQ8 and non-associated HLA-DQ9 alleles in complex with two disease-specific epitopes. Int. Immunol. 12, 1157–1166 (2000).
Molberg, Ø. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature Med. 4, 713–717 (1998); erratum 4, 974 (1998). The first paper to show that TG2 can post-translationally modify gluten peptides by deamidation and that T cells of the coeliac lesion preferentially recognize deamidated gluten peptides.
van de Wal, Y. et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998).
Mazzarella, G. et al. Gliadin activates HLA class I-restricted CD8+ T cells in celiac disease intestinal mucosa and induces the enterocyte apoptosis. Gastroenterology 134, 1017–1027 (2008).
Troncone, R. et al. In siblings of celiac children, rectal gluten challenge reveals gluten sensitization not restricted to celiac HLA. Gastroenterology 111, 318–324 (1996).
Maiuri, L. et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362, 30–37 (2003). This paper reports the ability of the 31–43 α-gliadin peptide, which is not recognized by T cells, to mediate innate immune effects in the coeliac mucosa.
Hüe, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004). The first paper to show that gluten can induce expression of MICs by IECs.
Barone, M. V. et al. Growth factor-like activity of gliadin, an alimentary protein: implications for coeliac disease. Gut 56, 480–488 (2007).
Terrazzano, G. et al. Gliadin regulates the NK-dendritic cell cross-talk by HLA-E surface stabilization. J. Immunol. 179, 372–381 (2007).
Cinova, J. et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J. Clin. Immunol. 27, 201–209 (2007).
Nikulina, M., Habich, C., Flohe, S. B., Scott, F. W. & Kolb, H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J. Immunol. 173, 1925–1933 (2004).
Junker, Y., Leffler, D. A., Wieser, H. & Schuppan, D. Gliadin activates monocytes, macrophages and dendritic cells in vitro and in vivo via Toll like receptor 4. Gastroenterology 136, A468 (2009).
Lammers, K. M. et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135, 194–204. e3 (2008).
Doyle, H. A. & Mamula, M. J. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 22, 443–449 (2001).
Siegel, M. et al. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE 3, e1861 (2008). This paper shows for the first time that TG2 is not constitutively active in the intestine and can be activated on TLR3-induced tissue injury.
Hovhannisyan, Z. et al. The role of HLA-DQ8 β57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature 456, 534–538 (2008). This paper shows how lack of a negative charge at position β57 of an MHC class II molecule affects the TCR repertoire and amplifies the T cell response to gluten.
Vader, W. et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122, 1729–1737 (2002).
Vader, W. et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl Acad. Sci. USA 100, 12390–12395 (2003). This paper introduces the concept that there are thresholds for activation of gluten-specific T cells. According to this model, HLA-DQ expression and the available number of T cell-stimulatory gluten peptides are crucial limiting factors for coeliac disease development.
Ploski, R., Ek, J., Thorsby, E. & Sollid, L. M. On the HLA-DQ(α1*0501, β1*0201)-associated susceptibility in celiac disease: a possible gene dosage effect of DQB1*0201. Tissue Antigens 41, 173–177 (1993).
Karell, K. et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the european genetics cluster on celiac disease. Hum. Immunol. 64, 469–477 (2003).
Tollefsen, S. et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J. Clin. Invest. 116, 2226–2236 (2006).
Fallang, L. E. et al. Differences in the risk of celiac disease associated with HLA-DQ2.5 or HLA-DQ2.2 are related to sustained gluten antigen presentation. Nature Immunol. 10, 1096–1101 (2009). This paper shows that HLA-DQ2.5 is better at presenting gluten peptides to T cells over a prolonged period than HLA-DQ2.2. The differential risk for coeliac disease of these two HLA molecules is likely to be related to this phenomenon.
Todd, J. A., Bell, J. I. & McDevitt, H. O. HLA-DQβ gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329, 599–604 (1987).
Lee, K. H., Wucherpfennig, K. W. & Wiley, D. C. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nature Immunol. 2, 501–507 (2001).
Henderson, K. N. et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity 27, 23–34 (2007).
Baker, F. J., Lee, M., Chien, Y. H. & Davis, M. M. Restricted islet-cell reactive T cell repertoire of early pancreatic islet infiltrates in NOD mice. Proc. Natl Acad. Sci. USA 99, 9374–9379 (2002).
Kim, C. Y., Quarsten, H., Bergseng, E., Khosla, C. & Sollid, L. M. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc. Natl Acad. Sci. USA 101, 4175–4179 (2004).
Qiao, S. W. et al. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: importance of proline spacing and glutamine deamidation. J. Immunol. 175, 254–261 (2005).
Price, P. et al. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol. Rev. 167, 257–274 (1999).
van de Wal, Y. et al. Unique peptide binding characteristics of the disease-associated DQ(α1*0501, β1*0201) vs the non-disease-associated DQ(α1*0201, β1*0202) molecule. Immunogenetics 46, 484–492 (1997).
Henrickson, S. E. et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nature Immunol. 9, 282–291 (2008).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009).
Chen, Y. et al. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376, 177–180 (1995).
Mora, J. R. & von Andrian, U. H. Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity 21, 458–460 (2004).
Iliev, I. D., Matteoli, G. & Rescigno, M. The yin and yang of intestinal epithelial cells in controlling dendritic cell function. J. Exp. Med. 204, 2253–2257 (2007).
Faria, A. M. & Weiner, H. L. Oral tolerance. Immunol. Rev. 206, 232–259 (2005).
Husby, S., Mestecky, J., Moldoveanu, Z., Holland, S. & Elson, C. O. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J. Immunol. 152, 4663–4670 (1994).
Molberg, Ø. et al. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand. J. Immunol. 46, 103–109 (1997).
Jabri, B. et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 118, 867–879 (2000).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Maiuri, L. et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119, 996–1006 (2000).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Rev. Immunol. 6, 595–601 (2006).
Blanco, P., Palucka, A. K., Pascual, V. & Banchereau, J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 19, 41–52 (2008).
Monteleone, G. et al. Role of interferon α in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut 48, 425–429 (2001).
Di Sabatino, A. et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology 133, 1175–1187 (2007).
Ráki, M. et al. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology 131, 428–438 (2006).
Cammarota, G., Cuoco, L., Cianci, R., Pandolfi, F. & Gasbarrini, G. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. Lancet 356, 1494–1495 (2000).
Troncone, R. & Auricchio, S. Rotavirus and celiac disease: clues to the pathogenesis and perspectives on prevention. J. Pediatr. Gastroenterol. Nutr. 44, 527–528 (2007).
Benahmed, M. et al. Inhibition of TGF-β signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology 132, 994–1008 (2007).
Fina, D. et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57, 887–892 (2008).
Peluso, I. et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J. Immunol. 178, 732–739 (2007).
Perera, L. et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm. Bowel Dis. 13, 298–307 (2007).
Kasaian, M. T. et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16, 559–569 (2002).
Sollid, L. M., Molberg, Ø., McAdam, S. & Lundin, K. E. Autoantibodies in coeliac disease: tissue transglutaminase — guilt by association? Gut 41, 851–852 (1997).
Matysiak-Budnik, T. et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 205, 143–154 (2008).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors as regulators of immune responses. Nature Rev. Immunol. 8, 34–47 (2008).
Patey-Mariaud de Serre, N. et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant 29, 223–230 (2002).
Cuenod, B. et al. Classification of intractable diarrhea in infancy using clinical and immunohistological criteria. Gastroenterology 99, 1037–1043 (1990).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 102, 330–354 (1992).
Kutlu, T. et al. Numbers of T cell receptor (TCR) αβ+ but not of TcR γδ+ intraepithelial lymphocytes correlate with the grade of villous atrophy in coeliac patients on a long term normal diet. Gut 34, 208–214 (1993).
Black, K. E., Murray, J. A. & David, C. S. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J. Immunol. 169, 5595–5600 (2002).
de Kauwe, A. L. et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J. Immunol. 182, 7440–7450 (2009).
Yokoyama, S. et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc. Natl Acad. Sci. USA 106, 15849–15854 (2009).
Zhou, R., Wei, H., Sun, R., Zhang, J. & Tian, Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl Acad. Sci. USA 104, 7512–7515 (2007).
Louka, A. S. & Sollid, L. M. HLA in coeliac disease: unravelling the complex genetics of a complex disorder. Tissue Antigens 61, 105–117 (2003).
Roberts, A. I. et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 167, 5527–5530 (2001).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004). The first paper to provide a molecular basis for IL-15-induced killer activity in vivo and antigen-nonspecific killing of IECs in coeliac disease and the first to report, together with reference 30, on the role of NKG2D in coeliac disease pathogenesis.
Jabri, B. et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 17, 487–499 (2002).
Meresse, B. et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 203, 1343–1355 (2006). This paper shows genetic reprogramming of CTLs into NK cell-like cells and HLA-E induction in IECs in coeliac disease. It also provides a molecular basis for cytokine production and expansion of intraepithelial lymphocytes that do not recognize gluten peptides.
Tang, F. et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J. Exp. Med. 206, 707–719 (2009).
Werz, O. 5-lipoxygenase: cellular biology and molecular pharmacology. Curr. Drug Targets. Inflamm. Allergy 1, 23–44 (2002).
Halstensen, T. S., Scott, H. & Brandtzaeg, P. Intraepithelial T cells of the TcRγ/δ+ CD8− and Vδ1/Jδ1+ phenotypes are increased in coeliac disease. Scand. J. Immunol. 30, 665–672 (1989).
Wu, J., Groh, V. & Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J. Immunol. 169, 1236–1240 (2002).
Spada, F. M. et al. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191, 937–948 (2000).
Bhagat, G. et al. Small intestinal CD8+TCRγδ+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J. Clin. Invest. 118, 281–293 (2008).
Maki, M., Holm, K., Collin, P. & Savilahti, E. Increase in γ/δ T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut 32, 1412–1414 (1991).
Rosen, D. B. et al. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 173, 2470–2478 (2004).
Daum, S., Cellier, C. & Mulder, C. J. Refractory coeliac disease. Best Pract. Res. Clin. Gastroenterol. 19, 413–424 (2005).
Cellier, C. et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 114, 471–481 (1998). This paper identifies for the first time the phenotypic changes — that is, the loss of surface expression of the TCR — in intraepithelial lymphocytes of patients with refractory sprue.
Fehniger, T. A. et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193, 219–231 (2001).
Groh, V., Bruhl, A., El-Gabalawy, H., Nelson, J. L. & Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 100, 9452–9457 (2003).
Ogasawara, K. et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20, 757–767 (2004).
Fabris, P. et al. Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet 340, 548 (1992).
Marazuela, M. et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-α therapy. Clin. Endocrinol. 44, 635–642 (1996).
Lundin, K. E. et al. Gliadin-specific, HLA-DQ(α1*0501, β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178, 187–196 (1993).
Lundin, K. E., Scott, H., Fausa, O., Thorsby, E. & Sollid, L. M. T cells from the small intestinal mucosa of a DR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum. Immunol. 41, 285–291 (1994).
Zhernakova, A., van Diemen, C. C. & Wijmenga, C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nature Rev. Genet. 10, 43–55 (2009).
Dieterich, W. et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nature Med. 3, 797–801 (1997). This paper identifies TG2 as the dominant antigen recognized by autoantibodes of patients with coeliac disease.
Fritzler, M. & Wiik, A. Autoantibody Assays, Testing and Standardization (eds Rose, I. & Mackay, I.) (Elsevier Academic, Sydney, 2006).
Schellekens, G. A., de Jong, B. A., van den Hoogen, F. H., van de Putte, L. B. & van Venrooij, W. J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 101, 273–281 (1998).
Ruckert, R. et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J. Immunol. 165, 2240–2250 (2000).
Lorand, L. & Graham, R. M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature Rev. Mol. Cell Biol. 4, 140–156 (2003).
Molberg, Ø. et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur. J. Immunol. 31, 1317–1323 (2001).
Vader, L. W. et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 195, 643–649 (2002). This paper characterizes the enzyme specificity of TG2 and shows that the preferred substrate sequences are frequently found in proteins of wheat, rye and barley.
Dørum, S., Qiao, S. W., Sollid, L. M. & Fleckenstein, B. A quantitative analysis of transglutaminase 2-mediated deamidation of gluten peptides: implications for the T-cell response in celiac disease. J. Proteome Res. 8, 1748–1755 (2009).
Djilali-Saiah, I. et al. CTLA-4 gene polymorphism is associated with predisposition to coeliac disease. Gut 43, 187–189 (1998).
Trynka, G. et al. Coeliac disease associated risk variants in TNFAIP3 and REL implicate altered NF-κB signalling. Gut 58, 1078–1083 (2009).
Acknowledgements
We thank patients with coeliac disease and their family members for their support of our research. We also thank the present and former members of our laboratories for their contributions to the work cited. Thanks are especially extended to V. Abadie for help with preparation of the figures. The work was supported by the US National Institutes of Health (grants RO1DK063158, RO1DK58727, P30DK42086), the Research Council of Norway, the European Commission FP7 programme, the South-Eastern Norway Regional Health Authority, the Juvenile Diabetes Research Foundation and the Norwegian Foundation for Health and Rehabilitation.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Gluten
-
Wheat proteins that are not tolerated by people with coeliac disease. Similar proteins exist in barley and rye. Gluten consists of proline- and glutamine-rich gliadin and glutenin subcomponents.
- Villi
-
Projections into the lumen that have an outer layer that mainly consists of mature, absorptive enterocytes and also contain mucus-secreting goblet cells.
- Crypts
-
Tubular invaginations of the intestinal epithelium. At the base of the crypts there are paneth cells, which produce bactericidal defensins, and stem cells, which continuously divide and are the source of all intestinal epithelial cells.
- Regulatory T (TReg)cell
-
A specialized subpopulation of CD4+ T cells that can suppress the effector responses of other T cells. They are characterized by the expression of the transcription factor forkhead box P3 (FOXP3).
- Linkage disequilibrium
-
The nonrandom association of alleles at distinct loci owing to close physical proximity of the loci and a lack of recombination between them.
- T helper 1 cell
-
(TH1 cell). CD4+ T cells that produce interferon and tumour necrosis factor and support cell-mediated immunity.
- Mesenteric lymph nodes
-
Lymph nodes located at the base of the mesentery. They collect lymph (including cells and antigens) draining from the intestinal mucosa.
- Intraepithelial lymphocyte
-
(IEL). A T cell that resides in the basolateral side of the intestinal epithelium. IELs express either an αβ T cell receptor (TCR) or a γδ TCR.
- Interleukin-15
-
(IL-15). A pro-inflammatory cytokine that is trans-presented by the IL-15 receptor α-chain to neighbouring cells that express the IL-2 or IL-15 receptor β-chain and common γ-chain (γc). It is best known for its role in the development and/or survival of natural killer cells and memory CD8+ T cells. However, it is now recognized that IL-15 also enhances the effector functions of natural killer and cytotoxic T cells.
- Leader peptides
-
(Also known as signal sequences). Hydrophobic amino acid sequences that signal for proteins to translocate to the endoplasmic reticulum. The leader peptide is cleaved before a protein is transported from the cell.
- HLA-E
-
A human non-classical MHC class I molecule that is composed of the HLA-E heavy chain, β2-microglobulin, and a peptide that is often derived from the leader peptides of other MHC class I polypeptides or from certain microbial pathogens. HLA-E is recognized by CD94–NKG2 receptors.
- CD94–NKG2C
-
An activating C-type lectin natural killer cell receptor that is expressed by natural killer cells (under normal conditions) and some T cells (under pathological conditions).
- Polyinosinic–polycytidylic acid
-
(PolyI:C). A substance that is used as a mimic of viral double-stranded RNA.
- Non-obese diabetic (NOD) mice
-
A strain of mouse that spontaneously develops idiopathic autoimmune diabetes that closely resembles type 1 diabetes in humans and involves autoreactive T cell-mediated destruction of pancreatic β-islet cells. The main component of susceptibility is the MHC haplotype H2g7.
- Oral tolerance
-
Induction of peripheral immune tolerance by oral administration of antigen. Oral tolerance is now linked to the induction of forkhead box P3 (FOXP3)+ regulatory T cells.
- Lamina propria
-
The layer of mucosal tissue directly beneath the mucosal epithelial cell surface, in which effector cells for mucosal immunity reside.
- Plasmacytoid dendritic cell
-
(Plasmacytoid DC). An immature DC with a morphology that resembles that of a plasma cell. Plasmacytoid DCs produce large quantities of type I interferons (that is, interferon-α and interferon-β) after activation: for example, when stimulated through Toll-like receptors.
- Intraepithelial cytotoxic T lymphocytes
-
(Intraepithelial CTLs). Cytotoxic CD8+ T cells found in the epithelial layer that lines mucosal surfaces. Their main role is to maintain the integrity of the mucosa by eliminating infected epithelial cells.
- Immune complex
-
Complexes of antigen bound to antibody and, sometimes, components of the complement system. The levels of immune complexes are increased in many autoimmune disorders, in which they become deposited in tissues and cause tissue damage.
- NKG2D
-
(Natural killer group 2, member D). A co-activating C-type lectin natural killer cell receptor that is expressed by human cytotoxic T cells, innate-like T cells and NK cells.
Rights and permissions
About this article
Cite this article
Jabri, B., Sollid, L. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol 9, 858–870 (2009). https://doi.org/10.1038/nri2670
Issue Date:
DOI: https://doi.org/10.1038/nri2670
This article is cited by
-
Novel Bacteroides Vulgatus strain protects against gluten-induced break of human celiac gut epithelial homeostasis: a pre-clinical proof-of-concept study
Pediatric Research (2024)
-
Role of age in dynamics of autoantibodies in pediatric Celiac disease
Italian Journal of Pediatrics (2023)
-
Coronavirus disease 2019 (COVID-19) in pediatric patients with autoimmune disorders
European Journal of Pediatrics (2023)
-
Antigen-driven colonic inflammation is associated with development of dysplasia in primary sclerosing cholangitis
Nature Medicine (2023)