Key Points
-
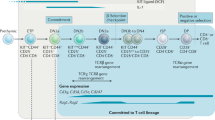
Directional cell migration into and within the post-natal thymus is intricately linked to the T-cell differentiation process.
-
Directional cell migration can be controlled in numerous ways, including: restricting the distribution of ligands for cell adhesion and traction, activating or inactivating receptors for cell adhesion and traction that are expressed by the migrating cells, and differentially localizing the sources of chemoattractant and/or repulsive signals.
-
Additional specificity is provided by expressing unique combinations of adhesion or chemoattractant receptors by progenitors at different stages of development.
Abstract
Similar to all haematopoietic lineages, T cells must be replenished throughout life — a process that is the main function of the thymus. New progenitors are recruited to leave the bloodstream and enter the thymus, then to migrate in a defined pattern within the thymus during differentiation, and finally to return to the blood after maturation. Thereby, directional migration is intrinsically linked to all stages of T-cell differentiation. This review focuses on what is known and what is unknown about the signals that support this migration process in the mouse model of post-natal thymocyte differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Osterfield, M., Kirschner, M. W. & Flanagan, J. G. Graded positional information: interpretation for both fate and guidance. Cell 113, 425–428 (2003).
Watt, F. M. Stem cell fate and patterning in mammalian epidermis. Curr. Opin. Genet. Dev. 11, 410–417 (2001).
Marshman, E., Booth, C. & Potten, C. S. The intestinal epithelial stem cell. Bioessays 24, 91–98 (2002).
Jegou, B. The Sertoli-germ cell communication network in mammals. Int. Rev. Cytol. 147, 25–96 (1993).
Nilsson, S. K. et al. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood 101, 856–862 (2003).
Kondo, M., Weissman, I. L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Igarashi, H., Gregory, S. C., Yokota, T., Sakaguchi, N. & Kincade, P. W. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17, 117–130 (2002). This manuscript indicates that the earliest lymphoid progenitor found in bone marrow seems to be marked by transcription from the recombinase-activating gene 1 ( RAG1 ) locus.
Allman, D. et al. Thymopoiesis independent of common lymphoid progenitors. Nature Immunol. 4, 168–174 (2003). This work describes what seems to be the earliest T-cell-lineage progenitor found in the thymus, and shows that it differs from the canonical common lymphoid progenitor.
Ikawa, T., Kawamoto, H., Fujimoto, S. & Katsura, Y. Commitment of common T/natural killer (NK) progenitors to unipotent T and NK progenitors in the murine fetal thymus revealed by a single progenitor assay. J. Exp. Med. 190, 1617–1626 (1999). This paper shows that the first requirement for Notch1 in the thymus seems to include repression of the default B-cell-lineage fate and/or to specify T-cell-lineage differentiation.
Ardavin, C., Wu, L., Li, C. L. & Shortman, K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 362, 761–763 (1993).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Moore, T. A. & Zlotnik, A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood 86, 1850–1860 (1995).
Laudanna, C., Kim, J. Y., Constantin, G. & Butcher, E. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186, 37–46 (2002).
Ruiz, P., Wiles, M. V. & Imhof, B. A. α6 integrins participate in pro-T cell homing to the thymus. Eur. J. Immunol. 25, 2034–2041 (1995).
Wu, L., Kincade, P. W. & Shortman, K. The CD44 expressed on the earliest intrathymic precursor population functions as a thymus homing molecule but does not bind to hyaluronate. Immunol. Lett. 38, 69–75 (1993).
Champion, S., Imhof, B. A., Savagner, P. & Thiery, J. P. The embryonic thymus produces chemotactic peptides involved in the homing of hemopoietic precursors. Cell 44, 781–790 (1986).
Bleul, C. C. & Boehm, T. Chemokines define distinct microenvironments in the developing thymus. Eur. J. Immunol. 30, 3371–3379 (2000).
Wilkinson, B., Owen, J. J. & Jenkinson, E. J. Factors regulating stem cell recruitment to the fetal thymus J. Immunol. 162, 3873–3881 (1999).
Wurbel, M. A. et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood 98, 2626–2632 (2001).
Suniara, R. K., Jenkinson, E. J. & Owen, J. J. Studies on the phenotype of migrant thymic stem cells. Eur. J. Immunol. 29, 75–80 (1999).
Kawakami, N. et al. Roles of integrins and CD44 on the adhesion and migration of fetal liver cells to the fetal thymus. J. Immunol. 163, 3211–3216 (1999).
Kincade, P. W. et al. Nature or nurture? Steady-state lymphocyte formation in adults does not recapitulate ontogeny. Immunol. Rev. 187, 116–125 (2002).
Owen, J. J. & Ritter, M. A. Tissue interaction in the development of thymus lymphocytes. J. Exp. Med. 129, 431–442 (1969).
Moore, M. A. & Owen, J. J. Experimental studies on the development of the thymus. J. Exp. Med. 126, 715–726 (1967).
Le, D. N. M. & Jotereau, F. V. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J. Exp. Med. 142, 17–40 (1975).
Rossiter, H., Alon, R. & Kupper, T. S. Selectins, T-cell rolling and inflammation. Mol. Med. Today 3, 214–222 (1997).
Ushiki, T. & Takeda, M. Three-dimensional ultrastructure of the perivascular space in the rat thymus. Arch. Histol. Cytol. 60, 89–99 (1997).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Lepique, A. P., Palencia, S., Irjala, H. & Petrie, H. T. Characterization of vascular adhesion molecules that may facilitate progenitor homing in the post–natal mouse thymus. Clin. Dev. Immunol. 10, 27–33 (2003).
Dunon, D. et al. Ontogeny of the immune system: γδ and αβ T cells migrate from thymus to the periphery in alternating waves. J. Exp. Med. 186, 977–988 (1997).
Lind, E. F., Prockop, S. E., Porritt, H. E. & Petrie, H. T. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J. Exp. Med. 194, 127–134 (2001).
Foss, D. L., Donskoy, E. & Goldschneider, I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J. Exp. Med. 193, 365–374 (2001).
Springer, T. A. Adhesion receptors of the immune system. Nature 346, 425–434 (1990). This study shows that recruitment of new progenitors from the blood to the post-natal thymus is a regulated periodic process, rather than occurring constitutively.
Savino, W., Villa-Verde, D. M. & Lannes-Vieira, J. Extracellular matrix proteins in intrathymic T-cell migration and differentiation? Immunol. Today 14, 158–161 (1993).
Kishimoto, H. et al. Differing roles for B7 and intercellular adhesion molecule-1 in negative selection of thymocytes. J. Exp. Med. 184, 531–537 (1996).
Sawada, M. et al. Expression of VLA-4 on thymocytes. Maturation stage-associated transition and its correlation with their capacity to adhere to thymic stromal cells. J. Immunol. 149, 3517–3524 (1992).
Crisa, L. et al. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J. Exp. Med. 184, 215–228 (1996).
Wadsworth, S., Halvorson, M. J. & Coligan, J. E. Developmentally regulated expression of the β4 integrin on immature mouse thymocytes. J. Immunol. 149, 421–428 (1992).
Prockop, S. E. et al. Stromal cells provide the matrix for migration of early lymphoid progenitors through the thymic cortex. J. Immunol. 169, 4354–4361 (2002).
Lannes-Vieira, J., Dardenne, M. & Savino, W. Extracellular matrix components of the mouse thymus micro-environment: ontogenetic studies and modulation by glucocorticoid hormones. J. Histochem. Cytochem. 39, 1539–1546 (1991).
Kim, M. G. et al. Epithelial cell-specific laminin 5 is required for survival of early thymocytes. J. Immunol. 165, 192–201 (2000).
Harman, B. C., Jenkinson, E. J. & Anderson, G. Microenvironmental regulation of Notch signalling in T cell development. Semin. Immunol. 15, 91–97 (2003).
Schmitt, T. M. & Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002). This work shows that the Notch ligand delta-like 1 is sufficient to specify the differentiation of T-cell-lineage progenitors in stromal cell cultures in vitro.
Savino, W., Mendes-da-Cruz, D. A., Silva, J. S., Dardenne, M. & Cotta-de-Almeida, V. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 23, 305–313 (2002).
Norment, A. M. & Bevan, M. J. Role of chemokines in thymocyte development. Semin. Immunol. 12, 445–455 (2000).
Pelletier, A. J. et al. Presentation of chemokine SDF-1α by fibronectin mediates directed migration of T cells. Blood 96, 2682–2690 (2000). This manuscript shows that directional T-cell migration does not require a gradient of chemokines, but rather an initial polarizing event together with persistence of the chemokine signal.
Youn, B. S. et al. Role of the CC chemokine receptor 9/TECK interaction in apoptosis. Apoptosis 7, 271–276 (2002).
Bousso, P., Bhakta, N. R., Lewis, R. S. & Robey, E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science 296, 1876–1880 (2002).
Yanagawa, Y., Iwabuchi, K. & Onoe, K. Enhancement of stromal cell-derived factor-1α-induced chemotaxis for CD4/8 double-positive thymocytes by fibronectin and laminin in mice. Immunology 104, 43–49 (2001).
Uehara, S., Song, K., Farber, J. M. & Love, P. E. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3highCD69+ thymocytes and γδTCR+ thymocytes preferentially respond to CCL25. J. Immunol. 168, 134–142 (2002).
Shortman, K., Egerton, M., Spangrude, G. J. & Scollay, R. The generation and fate of thymocytes. Semin. Immunol. 2, 3–12 (1990).
Norment, A. M., Bogatzki, L. Y., Gantner, B. N. & Bevan, M. J. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is upregulated following pre-TCR signaling. J. Immunol. 164, 639–648 (2000).
Wurbel, M. A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Vicari, A. P. et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 7, 291–301 (1997).
Campbell, J. J., Pan, J. & Butcher, E. C. Cutting edge: developmental switches in chemokine responses during T cell maturation. J. Immunol. 163, 2353–2357 (1999). This work shows several stage-specific distinctions in the responsiveness of developing thymocytes to various chemokines.
Suzuki, G. et al. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J. Immunol. 162, 5981–5985 (1999). This work indicates that movement from the cortex to the medulla is an active event requiring G protein-coupled signal transduction.
Chantry, D. et al. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3+, CD4+, CD8low thymocytes. Blood 94, 1890–1898 (1999).
Kremer, L. et al. The transient expression of CC chemokine receptor 8 in thymus identifies a thymocyte subset committed to become CD4+ single-positive T cells. J. Immunol. 166, 218–225 (2001).
Fukui, Y. et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 412, 826–831 (2001).
Lu, T. T. & Cyster, J. G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297, 409–412 (2002).
Suzuki, G. et al. Loss of SDF-1 receptor expression during positive selection in the thymus. Int. Immunol. 10, 1049–1056 (1998).
Ueno, T. et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity 16, 205–218 (2002).
Shi, G. X., Harrison, K., Wilson, G. L., Moratz, C. & Kehrl, J. H. RGS13 regulates germinal center B lymphocytes responsiveness to CXC chemokine ligand (CXCL)12 and CXCL13. J. Immunol. 169, 2507–2515 (2002).
Wilkinson, D. G. Multiple roles of EPH receptors and ephrins in neural development. Nature Rev. Neurosci. 2, 155–164 (2001).
Vergara-Silva, A., Schaefer, K. L. & Berg, L. J. Compartmentalized Eph receptor and ephrin expression in the thymus. Gene Expr. Patterns 2, 261–265 (2002). This work, although largely descriptive, indicates a potential role for canonical mediators of cell repulsion (ephrins) in directing cell location in the thymus.
Toro, I. & Olah, I. Penetration of thymocytes into the blood circulation. J. Ultrastruct. Res. 17, 439–451 (1967).
Bhalla, D. K. & Karnovsky, M. J. Surface morphology of mouse and rat thymic lymphocytes: an in situ scanning electron microscopic study. Anat. Rec. 191, 203–220 (1978).
Miyasaka, M., Pabst, R., Dudler, L., Cooper, M. & Yamaguchi, K. Characterization of lymphatic and venous emigrants from the thymus. Thymus 16, 29–43 (1990).
Sainte-Marie, G. & Leblond, C. P. Elaboration of a model for the formation of lymphocytes in the thymic cortex of young adult rats. Blood 26, 765–783 (1965).
Chaffin, K. E. & Perlmutter, R. M. A pertussis toxin-sensitive process controls thymocyte emigration. Eur. J. Immunol. 21, 2565–2573 (1991). This paper was one of the first to show that G protein-coupled signals are required for T-cell differentiation in the thymus, and indicates that this process is required for the export of newly generated T cells as well.
Poznansky, M. C. et al. Thymocyte emigration is mediated by active movement away from stroma-derived factors. J. Clin. Invest. 109, 1101–1110 (2002).
Zaitseva, M. et al. Stromal-derived factor 1 expression in the human thymus. J. Immunol. 168, 2609–2617 (2002).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Cook, D. N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Chensue, S. W. et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193, 573–584 (2001).
Acknowledgements
I would like to express my sincere thanks to Y. Takahama, P. Love and J. Cyster for helpful discussions before submission of the manuscript.
Author information
Authors and Affiliations
Related links
Glossary
- LINEAGE POTENTIAL
-
The lineages that a given progenitor can differentiate into, given the appropriate conditions. This does not necessarily indicate a biologically relevant outcome — that is, the fact that a given progenitor can be induced to generate cells of a specific lineage does not mean that it normally generates cells of that lineage.
- LINEAGE COMMITMENT
-
Irreversible differentiation into a single lineage, concurrent with loss of other lineage potential.
- HIGH ENDOTHELIAL VENULES
-
Specialized blood vessels with columnar endothelium that facilitate constitutive exchange of lymphocytes between the circulation and secondary lymphoid organs.
- POSITIVE SELECTION
-
The maturation of immature CD4+CD8+ precursor thymocytes induced by T-cell receptor (TCR) signals that result from binding to self-peptide–MHC ligands on thymic epithelial cells. This process selects thymocytes that express TCRs that can interact with self-MHC molecules.
Rights and permissions
About this article
Cite this article
Petrie, H. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol 3, 859–866 (2003). https://doi.org/10.1038/nri1223
Issue Date:
DOI: https://doi.org/10.1038/nri1223
This article is cited by
-
microRNA-449a modulates medullary thymic epithelial cell differentiation
Scientific Reports (2017)
-
Progenitor T-cell differentiation from hematopoietic stem cells using Delta-like-4 and VCAM-1
Nature Methods (2017)
-
CCR6 supports migration and differentiation of a subset of DN1 early thymocyte progenitors but is not required for thymic nTreg development
Immunology & Cell Biology (2014)
-
Tracking migration during human T cell development
Cellular and Molecular Life Sciences (2014)
-
Role of C-C chemokine receptor type 7 and its ligands during neuroinflammation
Journal of Neuroinflammation (2012)