Abstract
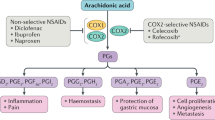
Following the withdrawal of rofecoxib and valdecoxib, the discussion concerning selective cyclo-oxygenase (COX)-2 inhibitors was often characterized more by emotions than scientific evidence. In fact, the original rationale of these substances is still valid, in that selective COX2 inhibitors cause significantly fewer severe side effects in the gastrointestinal tract than traditional NSAIDs. Off-label long-term use of COX2 inhibitors in patients with a history of colorectal adenomas in well-designed, placebo-controlled trials showed that treatment with these agents is associated with an increased rate of cardiovascular adverse effects. Other studies have shown that both COX2 inhibitors and NSAIDs are associated with a similar cardiovascular risk, suggesting that there is presently no rationale for a further differentiation of these groups of drugs in terms of cardiovascular toxicity. Referring to the current debate, potential mechanisms underlying cardiovascular adverse effects associated with the long-term use of COX2 inhibitors and NSAIDs are discussed. Moreover, this Review summarizes the pharmacology of COX2 inhibitors with emphasis on their different pharmacokinetic characteristics.
Key Points
-
Cyclo-oxygenase (COX)-2 inhibitors significantly reduce gastrointestinal adverse effects associated with long-term use of traditional NSAIDs
-
COX2 inhibitors and NSAIDs are associated with a similar cardiovascular risk
-
In some individuals high-dose naproxen (500 mg twice daily) might have a neutral effect or confer a small cardioprotection by virtue of a >95% COX1 inhibition
-
Permanent blockade of COX2-dependent prostaglandins (i.e. cardiorenal hypothesis) rather then an imbalance between COX1-derived and COX2-derived prostanoids is the currently most plausible explanation for the cardiovascular hazard conferred by COX2 inhibitors and NSAIDs
-
Despite newly defined contraindications and warnings, COX2 inhibitors and NSAIDs still remain important tools in pain therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action of aspirin-like drugs. Nat New Biol 231: 232–235
Masferrer JL et al. (1990) Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest 86: 1375–1379
Xie W et al. (1991) Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA 88: 2692–2696
Beiche F et al. (1996) Upregulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett 390: 165–169
FitzGerald GA and Patrono C (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345: 433–442
Hinz B and Brune K (2002) Cyclooxygenase-2—ten years later. J Pharmacol Exp Ther 300: 367–375
Flower RJ (2003) The development of COX2 inhibitors. Nat Rev Drug Discov 2: 179–191
Patrignani P et al. (1997) Differential inhibition of human prostaglandin endoperoxide synthase-1 and -2 by nonsteroidal anti-inflammatory drugs. J Physiol Pharmacol 48: 623–631
Tegeder I et al. (1999) Comparison of inhibitory effects of meloxicam and diclofenac on human thromboxane biosynthesis after single doses and at steady state. Clin Pharmacol Ther 65: 533–544
Hinz B et al. (2003) Aceclofenac spares cyclooxygenase 1 as a result of limited but sustained biotransformation to diclofenac. Clin Pharmacol Ther 74: 222–235
Luong C et al. (1996) Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol 3: 927–933
Brune K and Lanz R (1985) Pharmacokinetics of non-steroidal anti-inflammatory drugs. In Handbook of inflammation, Vol. 5. The Pharmacology of Inflammation, 413–449 (Eds Bonta IL et al.) Amsterdam: Elsevier Science Publishers
Werner U et al. (2002) Investigation of the pharmacokinetics of celecoxib by liquid chromatography-mass spectrometry. Biomed Chromatogr 16: 56–60
Werner U et al. (2003) Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin Pharmacol Ther 74: 130–137
Hinz B et al. (2006) More pronounced inhibition of cyclooxygenase-2, increase of blood pressure and decrease of heart rate by treatment with diclofenac compared with celecoxib and rofecoxib. Arthritis Rheum 54: 282–291
Rodrigues AD et al. (2003) Absorption, metabolism, and excretion of etoricoxib, a potent and selective cyclooxygenase-2 inhibitor, in healthy male volunteers. Drug Metab Dispos 31: 224–232
Scott G et al. (2004) Pharmacokinetics of lumiracoxib in plasma and synovial fluid. Clin Pharmacokinet 43: 467–478
Esser R et al. (2005) Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol 144: 538–550
Bombardier C et al. (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 343: 1520–1528
Schnitzer TJ et al. (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 364: 665–674
Silverstein FE et al. (2000) Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. J Am Med Assoc 284: 1247–1255
Juni P et al. (2002) Are selective COX2 inhibitors superior to traditional non-steroidal anti-inflammatory drugs? BMJ 324: 1287–1288
Laine L et al. (2007) Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomized comparison. Lancet 369: 465–473
Chan FK et al. (2002) Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 347: 2104–2110
Lai KC et al. (2005) Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med 118: 1271–1278
Maiden L et al. (2005) A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 128: 1172–1178
Goldstein JL et al. (2005) Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 3: 133–141
Picado C (2006) Mechanisms of aspirin sensitivity. Curr Allergy Asthma Rep 6: 198–202
Dahlen B et al. (2001) Celecoxib in patients with asthma and aspirin intolerance. The Celecoxib in Aspirin-Intolerant Asthma Study Group. N Engl J Med 344: 142
Stevenson DD and Simon RA (2001) Lack of cross-reactivity between rofecoxib and aspirin in aspirin-sensitive patients with asthma. J Allergy Clin Immunol 108: 47–51
Szczeklik A et al. (2001) Safety of a specific COX2 inhibitor in aspirin-induced asthma. Clin Exp Allergy 31: 219–225
Woessner KM et al. (2002) The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum 46: 2201–2206
Farkouh ME et al. (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 364: 675–684
Capone ML et al. (2004) Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation 109: 1468–1471
Bresalier RS et al. (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352: 1092–1102
Solomon SD et al. (2005) Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352: 1071–1080
Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B ; PreSAP Trial Investigators (2006) Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355: 885–895
Nussmeier NA et al. (2005) Complications of the COX2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352: 1081–1091
FDA (online 7 April 2005) FDA Announces Series of Changes to the Class of Marketed Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). [http://www.fda.gov/bbs/topics/news/2005/NEW01171.html] (accessed 20 August 2007)
EMEA (online 27 June 2005) Press release. European medicines agency concludes action on COX2 inhibitors. [http://www.emea.europa.eu/pdfs/human/press/pr/20776605en.pdf] (accessed 21 August 2007)
EMEA (online 17 October 2005) Press release. European Medicines Agency update on non-selective NSAIDs. [http://www.emea.europa.eu/pdfs/human/press/pr/29896405en.pdf] (accessed 21 August 2007)
ADAPT Research Group (2006) Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT). PLoS Clin Trials 1: e33
Kearney PM et al. (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1308
Hippisley-Cox J and Coupland C (2005) Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 330: 1366
Singh G et al. (2005) Both selective COX-2 inhibitors and non-selective NSAIDs increase the risk of acute myocardial infarction in patients with arthritis: selectivity is with the patient, not the drug class. Ann Rheum Dis 64 (Suppl III): 85
Cannon CP et al. (2006) Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the multinational etoricoxib and diclofenac arthritis long-term (MEDAL) programme: a randomised comparison. Lancet 368: 1771–1781
Flavahan NA (2007) Balancing prostanoid activity in the human vascular system. Trends Pharmacol Sci 28: 106–110
Rabausch K et al. (2005) Regulation of thrombomodulin expression in human vascular smooth muscle cells by COX2-derived prostaglandins. Circ Res 96: e1–e6
Reilly IA and FitzGerald GA (1987) Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 69: 180–186
McAdam BF et al. (1999) Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX2. Proc Natl Acad Sci USA 96: 272–277
Catella-Lawson F et al. (1999) Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther 289: 735–741
Schwartz JI et al. (2002) Comparison of rofecoxib, celecoxib, and naproxen on renal function in elderly subjects receiving a normal-salt diet. Clin Pharmacol Ther 72: 50–61
Whelton A et al. (2000) Renal safety and tolerability of celecoxib, a novel cyclooxygenase-2 inhibitor. Am J Ther 7: 159–175
Singh G et al. (2003) Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol 30: 714–719
Hansson L et al. (1998) Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 351: 1755–1762
Capone ML et al. (2005) Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol 45: 1295–1301
Catella-Lawson F et al. (2001) Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 345: 1809–1817
MacDonald TM and Wei L (2003) Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 361: 573–574
FDA (online 8 September 2006) Science background paper: concomitant use of ibuprofen and aspirin: potential for attenuation of the anti-platelet effect of aspirin. [http://www.fda.gov/cder/drug/infopage/ibuprofen/default.htm] (accessed 20 August 2007)
Hinz B et al. (2007) Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans – new insights into the pharmacology of an old analgesic. FASEB J 21: 2343–2351
Hinz B et al. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J, in press
Forman JP et al. (2005) Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension 46: 500–507
Chan AT et al. (2006) Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 113: 1578–1587
EMEA (2006) European Medicines Agency review concludes positive benefit-risk balance for non-selective NSAIDs. Doc. Ref. EMEA/413136/2006
Brune K and Hinz B (2004) Selective cyclooxygenase-2 inhibitors: similarities and differences. Scand J Rheumatol 33: 1–6
Therapeutic Goods Administration: Urgent advice regarding management of patients taking lumiracoxib [Prexige]. Safety alert. 13 August 2007 [http://www.tga.gov.au/alerts/prexige.htm]
Cheremina O et al. (2006) A validated high-performance liquid chromatographic assay for determination of lumiracoxib in human plasma. Biomed Chromatogr 20: 1033–1037
Esser R et al. (2005) Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol 144: 538–550
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Hinz has received consulting fees or honoraria from AstraZeneca and Novartis, and has received research support from Bayer. Dr. Renner has received research support from Merck Sharp & Dohme Limited, GlaxoSmithKline and Philip Morris USA. Dr. Brune has received consulting fees or honoraria from Bayer, Merck Sharp & Dohme Limited, and Novartis, and has received research support from Aventis, Bayer, Merck Sharp & Dohme Limited and Novartis.
Rights and permissions
About this article
Cite this article
Hinz, B., Renner, B. & Brune, K. Drug Insight: cyclo-oxygenase-2 inhibitors—a critical appraisal. Nat Rev Rheumatol 3, 552–560 (2007). https://doi.org/10.1038/ncprheum0619
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0619
This article is cited by
-
An observational study of the discrediting of COX-2 NSAIDs in Australia: Vioxx or class effect?
BMC Public Health (2011)
-
Managing adverse effects and drug–drug interactions of antiplatelet agents
Nature Reviews Cardiology (2011)
-
Using pharmacokinetic principles to optimize pain therapy
Nature Reviews Rheumatology (2010)
-
Absorption and distribution of etoricoxib in plasma, CSF, and wound tissue in patients following hip surgery—a pilot study
Naunyn-Schmiedeberg's Archives of Pharmacology (2010)