Abstract
Objective:
Our aim was to evaluate the safety of a silver–alginate-containing dressing to reduce peripherally inserted central catheter (PICC) infections in neonatal intensive care unit (NICU) patients.
Study Design:
Patients were randomized 3:1 to receive a patch containing silver, alginate and maltodextrin or standard of care. Patches were placed under the regular transparent retention dressing at the PICC exit site at insertion and were replaced with every dressing change at least every 2 weeks until PICC discontinuation. All study infants were monitored for adverse skin reactions.
Result:
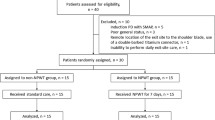
A total of 100 infants were followed up for 1922 person-days, including 75 subjects with 89 PICCs who received the patch. The median birth weight (1330 g) and median gestational age (30 weeks) was lower in the patch group when compared with the controls (P=0.001 and 0.005, respectively). Study patients received the patch with their PICC at a median age of 5 days; the patch stayed in place for a median of 13 days. We noted no adverse skin reactions and found no evidence that the patch alters the microbiology of PICC-associated infections.
Conclusion:
This pilot trial suggests that silver–alginate-coated dressings are skin safe and their inclusion in future trials aimed at reduction of PICC-associated bloodstream infections in the NICU should be considered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002; 110: 285–291.
Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004; 292: 2357–2365.
Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 2009; 301: 1231–1241.
Costello JM, Morrow DF, Graham DA, Potter-Bynoe G, Sandora TJ, Laussen PC . Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics 2008; 121: 915–923.
Ho KM, Litton E . Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother 2006; 58: 281–287.
Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001; 107: 1431–1436.
Cartwright DW . Central venous lines in neonates: a study of 2186 catheters. Arch Dis Child Fetal Neonatal Ed 2004; 89: F504–F508.
Khattak AZ, Ross RE, Arnold C, Tiffany N, Shoemaker CT . A randomized controlled trial of the safety of silver alginate (Algidex) patches in very low birth weight (VLBW) infants with central lines. Annual Meeting of the American Pediatric Society and Society for Pediatric Research, Hawaii, May 2008: 284. (abstract 665).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Silver. www.atsdr.cdc.gov/toxprofiles/phs146.html.
Eypasch E, Lefering R, Kum CK, Troidl H . Probability of adverse events that have not yet occurred: a statistical reminder. BMJ 1995; 311: 619–620.
Garland JS, Alex CP, Sevallius JM, Murphy DM, Good MJ, Volberding AM et al. Cohort study of the pathogenesis and molecular epidemiology of catheter-related bloodstream infection in neonates with peripherally inserted central venous catheters. Infect Control Hosp Epidemiol 2008; 29: 243–249.
Costa SF, Miceli MH, Anaissie EJ . Mucosa or skin as source of coagulase-negative staphylococcal bacteraemia? Lancet Infect Dis 2004; 4: 278–286.
Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH . Lack of enteral nutrition—effects on the intestinal immune system. J Surg Res 2005; 123: 8–16.
Barreau F, Ferrier L, Fioramonti J, Bueno L . Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 2004; 53: 501–506.
Lachman P, Yuen S . Using care bundles to prevent infection in neonatal and paediatric ICUs. Curr Opin Infect Dis 2009; 22: 224–228.
Saiman L . Strategies for prevention of nosocomial sepsis in the neonatal intensive care unit. Curr Opin Pediatr 2006; 18: 101–106.
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S.. Pediatrics 2002; 110: e51.
Edwards WH . Preventing nosocomial bloodstream infection in very low birth weight infants. Semin Neonatol 2002; 7: 325–333.
Clark R, Powers R, White R, Bloom B, Sanchez P, Benjamin Jr DK . Prevention and study of nosocomial sepsis in the NICU. J Perinatol 2004; 24: 446–453.
Acknowledgements
We thank the Vanderbilt NICU Research Fund for generous support of the study and DeRoyal, Inc. for providing the study patches. We also thank the Vanderbilt NICU nursing and physician staff members for their support of this project and Steven Steele for his assistance with data retrieval. We are grateful to Judy Aschner for review of this paper and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hill, M., Baldwin, L., Slaughter, J. et al. A silver–alginate-coated dressing to reduce peripherally inserted central catheter (PICC) infections in NICU patients: a pilot randomized controlled trial. J Perinatol 30, 469–473 (2010). https://doi.org/10.1038/jp.2009.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2009.190
Keywords
This article is cited by
-
Synthesis of silver nanoparticles utilizing various biological systems: mechanisms and applications—a review
Progress in Biomaterials (2020)
-
Prävention von Gefäßkatheter-assoziierten Infektionen bei Früh- und Neugeborenen
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2018)
-
Innovative dressing and securement of tunneled central venous access devices in pediatrics: a pilot randomized controlled trial
BMC Cancer (2017)
-
Eliminating Infections in the ICU: CLABSI
Current Infectious Disease Reports (2015)
-
New materials and devices for preventing catheter-related infections
Annals of Intensive Care (2011)