Abstract
Purpose
To compare retinal thickness (RT) measurement and segmentation performance of time domain (TD, Stratus) and spectral domain (SD) optical coherence tomography (OCT) devices (Cirrus, Spectralis) for imaging macular oedema (ME) secondary to branch retinal vein occlusion (BRVO).
Methods
In this study, 20 eyes of 20 consecutive patients with acute BRVO were included. A total of 18 unaffected fellow eyes served as control group. RT measurement was analysed in the five inner fields of the early-treatment diabetic retinopathy grid, and proportional segmentation errors were evaluated.
Results
Central millimetre thickness (CMT) showed a mean difference of −64, −74, and −18 μm (P<0.001) in the control group and −31 μm (P=0.107), −92 μm (P<0.001), and −105 μm (P=0.016) in the BRVO group, between Stratus and Cirrus, between Stratus and Spectralis, and between Cirrus and Spectralis, respectively. Mean RT showed the highest variability between different devices in the area most intensively affected by BRVO-related ME. In eyes with BRVO, 14.6% of Spectralis, 20% of Stratus, and 36.6% of Cirrus scans demonstrated moderate and severe segmentation errors.
Conclusion
RT measurement in eyes with BRVO, by TD and SD OCT, is compromised by a significant rate of segmentation errors. Deviations are most pronounced in the areas most severely affected by ME.
Similar content being viewed by others
Introduction
Retinal venous occlusion (RVO) is supposed to be the most common retinal vascular disorder following diabetic retinopathy. Branch retinal vein occlusion (BRVO), in particular, affects approximately 1% of the population over the age of 48 years.1 Acute BRVO has a characteristic clinical appearance with retinal haemorrhages, soft and hard exudates, retinal oedema, and dilated tortuous veins in a segmental distribution.2
Retinal thickness (RT) measurements are increasingly used to monitor macular oedema (ME),3, 4 and to assess the effectiveness of therapeutic interventions such as anti-angiogenic injections.5
With a vast spectrum of indications for treatment and the widespread presence of commercially available time domain (TD) and spectral domain (SD) optical coherence tomography (OCT) devices, the reliability and comparability of different OCT systems used in clinical practice have become a matter of interest. Although OCT software seems to be well adapted for healthy eyes,6, 7, 8 several studies have shown that the accuracy and comparability of the data is reduced in cases involving retinal pathology.8, 9, 10, 11, 12 It is hypothesised that the performance of OCT thickness measurements is also influenced by the type of macular pathology.8, 11
The SD-OCT devices Cirrus and Spectralis provide a high number of scans and measurement points for the automatic thickness analysis. Stratus OCT provides only six radial scans, and may therefore be associated with a higher rate of thickness measurement errors because of a higher need for interpolation.9
Generally, accurate detection of retinal boundaries, which is the base for precise thickness determination, mainly depends on the quality of OCT software algorithms.
To provide more insight into the reliability of OCT procedures in specific macular pathologies, it was the aim of this study to compare RT measurements and segmentation performances of different TD- and SD-OCT devices in eyes with ME secondary to BRVO.
Patients and methods
Patient inclusion
In this prospective, comparative, and cross-sectional clinical study, 38 eyes of 20 consecutive patients (12 men, 8 women) were included. A total of 20 eyes with BRVO served as study group, and the unaffected fellow eyes served as control group. Two eyes of the control group were excluded because of the presence of macular pathologies.
The study was approved by the local Institutional Ethics Committee and written informed consent was obtained from all participants before inclusion. The study followed the tenets of the Declaration of Helsinki. We certify that all applicable institutional and governmental regulations regarding the ethical use of human volunteers were followed during this research.
Main inclusion criterion for the study group was the confirmed diagnosis of treatment-naive acute BRVO with a history of less than 3 months. Exclusion criteria for both groups were history of glaucoma, aphakia, or presence of an anterior chamber intraocular lens, significant cataract, rubeosis iridis, ocular infections, and any history of additional retinal pathologies (that is, age-related macular degeneration, diabetic retinopathy, ME for other reasons than BRVO) or previous vitreoretinal surgery.
All patients underwent an initial standardised ophthalmic examination according to protocol, including measurement of best-corrected visual acuity with early-treatment diabetic retinopathy (ETDRS) procedures, and assessment of intraocular pressure, slit-lamp examination, ophthalmoscopy, and fluorescein angiography.
Optical coherence tomography imaging
After pupil dilation, OCT was performed in each eye by the same experienced examiner. Three different OCT technologies with the same settings described by Mylonas et al12 were used:
The conventional TD Stratus OCT system (Version 4.0.1-0056, Carl Zeiss Meditec, Dublin, CA, USA) offers an axial image resolution of ∼10 μm, a transversal resolution of 15 μm, and a scanning velocity of 400 A scans/second using the ‘fast macular thickness’ pattern. RT is calculated from six individual B scans, arranged in a radial pattern, and displayed in a grid following the recommendations of the ETDRS study.
The Cirrus SD-OCT (Version 3.0.0.6.4, Carl Zeiss Meditec) is a high-resolution SD-OCT device with an axial image resolution of 5 μm, a transversal resolution of 15 μm, and a scan velocity of up to 25 000 A scans/second. Raster scans cover an area of 6 × 6 mm2. Two different types of raster patterns (512 × 128 and 200 × 200) were used for RT measurements.
The Spectralis HRA+OCT system (Version 3.2.1.0, Heidelberg Engineering, Inc., Heidelberg, Germany) is a high-resolution SD-OCT, allowing simultaneous angiographic, infra-red, autofluorescenc, and OCT measurements. It has an axial image resolution of approximately 7 μm, a transversal resolution of 14 μm, and a scanning velocity up to 40 000 A scans/second. The Spectralis is capable to perform raster scans of the central macula using an eye-tracking system. A scanning pattern consisting of 37 B scans per raster was used in our study.
OCT grid projection and segmentation analysis criteria
Each OCT scan was displayed using a grid projection introduced by the authors of the ETDRS trial. To allow for comparative imaging, in BRVO and control eyes, individual fields were labelled as shown in Figure 1.
Optical coherence tomography grid projection introduced by the authors of the ETDRS trial. In this study, fields within the central 3 mm diameter were evaluated. In control group, fields were named as follows: CMT, central millimetre thickness; F2, superior inner macula; F3, temporal inner macula; F4, inferior inner macula; F5, nasal inner macula. In branch retinal vein occlusion (BRVO) eyes, fields were named as follows: CMT, central millimetre thickness; F2, area most intensively affected by BRVO-induced oedema; F3, temporal macula; F4, area relatively unaffected by BRVO-induced oedema; F5, nasal macula.
Mean RT of central millimetre thickness field (CMT) and of fields 2, 3, 4, and 5 (F2–F5) was assessed using the automatic thickness analysis of the described OCT devices. The measurements obtained by the devices with respect to their retinal location were then compared for differences and specific characteristics.
All individual scans were analysed by a masked observer certified for OCT-reading procedures by the Vienna Reading Center.
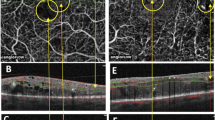
Apparent errors in the automated identification of the inner boundary of the neurosensory retina as well as the outer subretinal boundary, consistent with retinal pigment epithelium (RPE) for all sub-fields of the performed raster and radial scans were identified as segmentation errors.
Segmentation errors were classified into three categories according to Mylonas et al:12 negligible (<50 μm), moderate (50–200 μm), and severe (>200 μm) for segmentation errors in the length scan and negligible (<0.5 mm), moderate (0.5–2 mm), and severe (>2 mm) for artifacts in width scan. If more than one error was present in either direction, the documented error of the individual scan was defined as the severest error detected. Segments with a low signal because of ME, which are excluded from segmentation by the Spectralis OCT software (no segmentation line is visible in this area), were graded as segmentation errors based on the above-mentioned classification in width-scan direction. Figure 2 illustrates examples of all segmentation error categories.
Statistics
All data were entered into a Microsoft Excel spreadsheet. Statistical analysis was performed using Microsoft Excel and SPSS 12.0 software (SPSS, Chicago, IL, USA). The levels of statistical significance and correlation coefficients between the different devices were calculated using Wilcoxon's matched-pairs signed-rank test, χ2 test, and Pearson's correlation coefficients. Bland–Altman plots were used to identify a potential correlation between the differences and the mean results of two measurements. P-values of less than 0.05 were considered to be statistically significant. In the case of multiple comparisons, the Bonferroni–Holm correction was performed.
Results
Demographic data
The mean age of all participants was 68.7±7.8 years (range: 56–84 years). The mean duration of symptoms was at 1.9±1.5 months.
The oedema affected the superotemporal macular area in 15 eyes and the inferotemporal macular area in 5 eyes.
Mean signal intensity of analysed OCT images was 5±1.7 by Stratus OCT and 6±0.7 by Cirrus OCT (P=0.02).
Mean visual acuity was 52±11.5 and 68±4.2 ETDRS letters in the study group and in the control group, respectively (P<0.001).
Quantitative OCT analysis
Control group
In the control group, the Stratus data showed the lowest mean thickness values for all fields. The highest mean values were obtained by Spectralis (Table 1).
The mean difference in CMT between Stratus and Cirrus 512 × 128, between Stratus and Cirrus 200 × 200, and between Stratus and Spectralis was −64±24, −57±10, and −74±8.4 μm, respectively (P<0.001). The mean difference in CMT between Spectralis and Cirrus in the 200 × 200 pattern was 18±7.0 μm (P=0.001).
Consistently, there was a significant difference of mean CMT and macular thickness in all other fields (F2–F5) between the different OCT devices (P<0.05), except between the Cirrus 512 × 128 and the Cirrus 200 × 200 raster pattern (P>0.05).
Study group
In eyes with BRVO, the lowest mean CMT value (470 μm) was measured by Stratus and the highest mean RT value (572 μm) was measured by Spectralis, similar to the findings in the control group. Table 2 summarises the mean RT values for all OCT devices in the BRVO group.
The mean difference in CMT between Stratus and Cirrus 512 × 128, between Stratus and Cirrus 200 × 200, and between Stratus and Spectralis was −31±89 μm (n=19, P=0.107), −5±139 μm (n=19, P=0.882), and −92±87 μm (n=17, P<0.001), respectively. The mean difference in CMT between Spectralis and Cirrus 200 × 200 was −105±155 μm (n=16, P=0.016).
A statistically significant difference in CMT was only observed between Stratus and Spectralis (P<0.001) and between Cirrus and Spectralis (P=0.016). There was no statistically significant difference in CMT between Stratus, Cirrus 512 × 128, and Cirrus 200 × 200 (P>0.05).
With regard to mean RT values of the area most intensively affected by BRVO-induced ME (F2), a statistically significant difference was observed between all three OCT devices (P<0.01).
Only between Stratus and Spectralis did the thickness values of all five investigated fields differ significantly.
Correlation of retinal thickness measurements
In healthy eyes, the thickness values of all corresponding fields showed a high correlation between the different OCT devices (Pearson correlation coefficients ranging between r=0.51 and 0.98, Table 3).
In eyes with BRVO, correlations varied largely between the investigated OCT devices, ranging from −0.72 to +0.93. In the sub-field, most intensively affected by BRVO (F2), the Pearson correlation coefficients showed the lowest values, ranging between r=−0.72 and +0.81. As expected, correlations in F4 (unaffected area) were higher than in the other fields, ranging between r=0.54 and 0.93 (Table 4).
Figures 3 and 4 show Bland–Altman plots comparing mean values and mean differences of the CMT between the three OCT devices.
Segmentation performance
No substantial segmentation errors were found in Stratus scans of healthy eyes. However, in 25 of 2176 (1.1%) Cirrus scans and in 9 of 629 (1.4%) Spectralis scans, moderate or severe alignment errors were detected (P>0.05). In most cases, the segmentation errors were caused by incorrect identification of the internal limiting membrane due to a partial detachment of the vitreous body.
In eyes with BRVO, segmentation errors occurred in the performance of all OCT devices. Severe alignment errors were detected in 9 of 120 (7.5%) of Stratus scans, in 56 of 629 (8.9%) of Spectralis scans, and in 758 of 2432 (31.2%) of Cirrus scans (P<0.001, χ2 test between Cirrus 512 × 128 and Stratus, as well as between Cirrus 512 × 128 and Spectralis, P=0.617 between Stratus and Spectralis).
Moderate and severe segmentation errors were detected in 14.6% of Spectralis scans, 20% of Stratus scans, and 36.6% of Cirrus scans. With regard to moderate and severe segmentation errors, there was a significant difference between Cirrus 512 × 128 and Spectralis (P=0.012).
In all 99.6% of the described moderate and severe segmentation errors by Cirrus and 86% by Spectralis were caused by incorrect identification of the RPE. By Stratus, this boundary was only affected in 56.5% of moderate and severe segmentation errors.
Figure 5 illustrates the proportion of segmentation errors of varying degree.
Discussion
In this study, three different OCT devices comprising one TD- and two SD-OCT types were compared. The comparison was carried out by examining healthy eyes and eyes with ME because of BRVO, focusing on RT measurement and segmentation performance.
Regarding RT measurements, our results are similar to those of previous studies, which describe comparable baseline values in eyes with BRVO, assessed by a single OCT device (mainly Stratus)13, 14 and normal eyes.7, 15, 16 We therefore conclude that the population of our study group is representative for the pathology.
To our knowledge, this is the first comparative clinical study analysing RT measurements of TD- and SD-OCT devices in eyes with BRVO. Yamaike et al4 described the pathomorphological features of cystoid ME secondary to retinal vein occlusion using 3D OCT and Stratus OCT.5 In contrast to our study, they included eyes with both BRVO and CRVO, focusing on morphological features of affected retina without comparative RT measurements.
Mean RT differed significantly in healthy eyes throughout all investigated sub-fields between Spectralis, Cirrus, and Stratus OCT, whereas Spectralis provided reproducibly the highest and Stratus the lowest thickness values.
Obviously, the discrepancies between the three devices are the result of various segmentation algorithms. Although the segmentation algorithm of Stratus defines RT as distance between the inner/outer segment of the photoreceptor layer and the interrnal limiting membrane (ILM), the algorithm of Cirrus defines it as the distance between the RPE boundary and the ILM. Spectralis detects the RPE layer slightly deeper than the Cirrus software. This difference of segmentation localisation leads to reproducible and well-defined discrepancies of thickness values under physiological conditions when boundaries are intact.
In contrast to the highly correlating measurements in healthy eyes, there was a substantial variability of RT values in eyes with BRVO with lower correlation. The variability between the three devices was most pronounced in retinal areas most intensively affected by ME. Only F4, the sub-field that was relatively unaffected by ME, showed a higher corrrelation.
The fact, that there was a high correlation of all corresponding sub-fields in our control group, but a low correlation in eyes with BRVO, indicates a limited comparability of RT measurements between the three OCT devices in eyes affected by BRVO.
A limited interchangeability between Stratus and Cirrus OCT has also been demonstrated in other pathologies, such as neovascular age-related macular degeneration or diabetic ME.3, 12, 17
Several reasons for inaccurate measurements have been identified in recent studies. These include operator failure, decentration resulting from poor fixation, high foveal thickness standard deviation, and failure of accurate retinal boundary detection by the OCT software algorithms. In our study, all OCT images were taken by the same certified examiner, therefore operator failure could be ruled out.
Scan-by-scan analysis indicated that incorrect segmentation processes caused the errors in thickness measurement in affected eyes. Although almost no segmentation errors were found in the control group or unaffected sub-fields, the BRVO group, and particularly sub-fields, most intensively affected by ME, demonstrated segmentation errors of varying degree, depending on the OCT device. The most segmentation errors were detected in Cirrus scans and the fewest in Spectralis scans.
In all devices, segmentation errors occurred more frequently and more pronounced in areas affected by ME. Segmentation errors increased with the level of ME and were mainly caused by incorrect identification of the RPE. Overlying ME may lead to a lower scanning signal in the underlying areas. This may complicate the correct identification of limiting structures such as the RPE. The Spectralis software excludes areas with very low-signal quality because of extensive ME from analysis. In such case, no segmentation line is visible in the affected area. Many of the severe detection errors with this system were related to incorrect identification of the ILM because of a partial detachment of the vitreous.
As axial resolution and scanning speed are similar between Cirrus and Spectralis, the higher rate of segmentation errors with the Cirrus system seems to be a consequence of the different segmentation algorithms.
TD OCT systems may be expected to cause a higher rate of measurement failures because fewer measurement points are recorded. In our study, the TD Stratus OCT showed surprisingly few segmentation errors. It is also reported that Sadda et al11 investigated risk factors for measurement errors by Stratus OCT, and reported that retinal cysts and the diagnosis of a retinal vascular disease were less likely to cause severe segmentation errors than other pathologies, such as the presence of subretinal fluid.
In conclusion, the three examined OCT devices are not comparable for imaging eyes with ME secondary to BRVO. Overall, SD-OCT provided more detailed images and superior visualisation of intraretinal structures because of a high-resolution technology as reported previously.18
Nevertheless, in eyes with BRVO, SD Cirrus scans showed significantly more segmentation errors than (TD) Stratus scans. Accurate thickness measurement without segmentation errors is rather a matter of segmentation software than of OCT scanning technology.

References
Mitchell P, Smith W, Chang A . Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996; 114: 1243–1247.
Rehak J, Rehak M . Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res 2008; 33: 111–131.
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT . Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci 2008; 49 (10): 4290–4296.
Yamaike N, Tsujikawa A, Ota M, Sakamoto A, Kotera Y, Kita M et al. Three-dimensional imaging of cystoid macular edema in retinal vein occlusion. Ophthalmology 2008; 115: 355–362.
Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W et al. Intravitreal bevacizumab (Avastin) for macular edema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol 2009; 93: 452–456.
Legarreta JE, Gregori G, Punjabi OS, Knighton RW, Lalwani GA, Puliafito CA . Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging 2008; 39: S43–S49.
Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, Pang CP et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2008; 49: 4893–4897.
Ray R, Stinnett SS, Jaffe GJ . Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol 2005; 139: 18–29.
Ahlers C, Simader C, Geitzenauer W, Stock G, Stetson P, Dastmalchi S et al. Automatic segmentation in three-dimensional analysis of fibrovascular pigmentepithelial detachment using high-definition optical coherence tomography. Br J Ophthalmol 2008; 92: 197–203.
Leung CK, Chan WM, Chong KK, Chan KC, Yung WH, Tsang MK et al. Alignment artifacts in optical coherence tomography analysed images. Ophthalmology 2007; 114: 263–270.
Sadda SR, Wu Z, Walsh AC, Richine L, Dougall J, Cortez R et al. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology 2006; 113: 285–293.
Mylonas G, Ahlers C, Malamos P, Golbaz I, Deak G, Schütze C et al. Comparison of retinal thickness measurements and segmentation performance of four different spectral and time domain OCT devices in neovascular age-related macular degeneration. Br J Ophthalmol 2009; 93: 1453–1460.
Cheng KC, Wu WC . Intravitreal triamcinolone acetonide for patients with macular oedema due to branch retinal vein occlusion. Kaohsiung J Med Sci 2006; 22: 321–330.
Kreutzer TC, Alge CS, Wolf AH, Kook D, Burger J, Strauss R et al. Intravitreal bevacizumab for the treatment of macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol 2008; 92: 351–355.
Forte R, Cennamo GL, Finelli ML, de Crecchio G . Comparison of time domain Stratus OCT and spectral domain SLO/OCT for assessment of macular thickness and volume. Eye 2009; 23: 2071–2078.
Paunescu LA, Schuman JS, Price LL, Stark PC, Beaton S, Ishikawa H et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci 2004; 45: 1716–1724.
Kiernan DF, Hariprasad SM, Chin EK, Kiernan CL, Rago J, Mieler WF . Prospective comparison of cirrus and stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol 2009; 147: 267–275.
Pournaras JA, Erginay A, Lazrak Z, Gaudric A, Massin P . Spectral domain optical coherence tomography in diabetic macular oedema. Ophthalmic Surg Lasers Imaging 2009; 40: 548–553.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented at the ARVO Congress 2009 in Fort Lauderdale, FL, USA.
Rights and permissions
About this article
Cite this article
Matt, G., Sacu, S., Buehl, W. et al. Comparison of retinal thickness values and segmentation performance of different OCT devices in acute branch retinal vein occlusion. Eye 25, 511–518 (2011). https://doi.org/10.1038/eye.2010.216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.216
Keywords
This article is cited by
-
Comparison of retinal thickness measurements among four different optical coherence tomography devices
Scientific Reports (2024)
-
Automated retinal boundary segmentation of optical coherence tomography images using an improved Canny operator
Scientific Reports (2022)
-
Impact of manual correction over automated segmentation of spectral domain optical coherence tomography
International Journal of Retina and Vitreous (2020)
-
Branch Retinal Vein Occlusion: Treatment Outcomes According to the Retinal Nonperfusion Area, Clinical Subtype, and Crossing Pattern
Scientific Reports (2019)
-
Overestimation of subfoveal choroidal thickness by measurement based on horizontally compressed optical coherence tomography images
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)





 ) segmentation errors.
) segmentation errors.

