Abstract
Background/Objectives:
Subjects with type 2 diabetes (T2D) and their nondiabetic first-degree relatives (REL) have increased risk of cardiovascular disease (CVD). Postprandial triglyceridemia (PPL), influenced by diet, is an independent risk factor for CVD. Dietary fat elicits increased PPL in T2D compared with nondiabetic controls, but our knowledge of PPL responses to fat in REL is sparse. Our aim was to test the hypothesis that REL respond to a monounsaturated fatty acid (MUFA) challenge with a higher PPL response compared with controls who have no family history of T2D (CON) and that MUFAs exert a differential impact on incretin responses and on the expression of genes involved in carbohydrate and lipid metabolism in muscle and adipose tissues of REL and CON.
Subjects/Methods:
A total of 17 REL and 17 CON consumed a meal with 72 energy percent derived from MUFAs (macadamia nut oil). Plasma triglycerides, free fatty acids, insulin, glucose, glucagon-like peptide 1, glucose-dependent insulintropic peptide and ghrelin were measured at baseline and regular intervals until 4 h postprandially. Muscle and adipose tissue biopsies were collected at baseline and at 210 min after the meal.
Results:
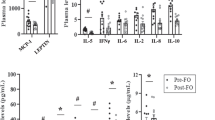
The MUFA-rich meal did not elicit different responses (P>0.05) in PPL, insulin, glucose, incretins or ghrelin in REL and CON. Several genes were differentially regulated in muscle and adipose tissues of REL and CON.
Conclusions:
A MUFA-rich meal elicits similar PPL, insulin and incretin responses in REL and CON. MUFAs have a differential impact on gene expression in muscle and adipose tissues in a pattern pointing toward early defects in lipid metabolism in REL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sarlund H Coronary heart disease and its risk factors in first-degree relatives of non-insulin-dependent diabetic and non-diabetic subjects. University of Kuopio: Kuopio, Finland, 1987. original report 6.
Karpe F . Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med 1999; 246: 341–355.
Kolovou GD, Mikhailidis DP, Nordestgaard BG, Bilianou H, Panotopoulos G . Definition of postprandial lipaemia. Curr Vasc Pharmacol 2011; 9: 292–301.
Lairon D, Defoort C . Effects of nutrients on postprandial lipemia. Curr Vasc Pharmacol 2011; 9: 309–312.
Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr 1999; 69: 1135–1143.
Thomsen C, Storm H, Holst JJ, Hermansen K . Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr 2003; 77: 605–611.
Rivellese AA, De Natale C, Di Marino L, Patti L, Iovine C, Coppola S et al. Exogenous and endogenous postprandial lipid abnormalities in type 2 diabetic patients with optimal blood glucose control and optimal fasting triglyceride levels. J Clin Endocrinol Metab 2004; 89: 2153–2159.
Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA . Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med 1999; 131: 27–31.
Kriketos A, Milner KL, Denyer G, Campbell L . Is postprandial hypertriglyceridaemia in relatives of type 2 diabetic subjects a consequence of insulin resistance? Eur J Clin Invest 2005; 35: 117–125.
Peake PW, Kriketos AD, Campbell LV, Charlesworth JA . Response of the alternative complement pathway to an oral fat load in first-degree relatives of subjects with type II diabetes. Int J Obes (Lond) 2005; 29: 429–435.
Normand-Lauziere F, Frisch F, Labbe SM, Bherer P, Gagnon R, Cunnane SC et al. Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PLoS One 2010; 5: e10956.
Johanson EH, Jansson PA, Gustafson B, Lonn L, Smith U, Taskinen MR et al. Early alterations in the postprandial VLDL1 apoB-100 and apoB-48 metabolism in men with strong heredity for type 2 diabetes. J Intern Med 2004; 255: 273–279.
Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV . Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes 2007; 56: 2046–2053.
Madec S, Corretti V, Santini E, Ferrannini E, Solini A . Effect of a fatty meal on inflammatory markers in healthy volunteers with a family history of type 2 diabetes. Br J Nutr 2011; 106: 364–368.
Pietraszek A, Hermansen K, Pedersen SB, Langdahl LL, Holst JJ, Gregersen S . Effects of a meal rich in medium-chain saturated fat on postprandial lipemia in relatives of type 2 diabetics. Nutrition 2013; 29: 1000–1006.
Deacon CF, Nauck MA, Meier J, Hucking K, Holst JJ . Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000; 85: 3575–3581.
Benjamini Y, Hochberg Y . Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 1995; 57: 289–300.
Kendall CW, Josse AR, Esfahani A, Jenkins DJ . Nuts, metabolic syndrome and diabetes. Br J Nutr 2010; 104: 465–473.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290.
Nyholm B, Walker M, Gravholt CH, Shearing PA, Sturis J, Alberti KG et al. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of Type II (non-insulin-dependent) diabetic parents: evidence of several aberrations. Diabetologia 1999; 42: 1314–1323.
Ostergard T, Hansen TK, Nyholm B, Gravholt CH, Djurhuus CB, Hosoda H et al. Circulating ghrelin concentrations are reduced in healthy offspring of Type 2 diabetic subjects, and are increased in women independent of a family history of Type 2 diabetes. Diabetologia 2003; 46: 134–136.
Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF . Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 2010; 82: 149–154.
Lobo S, Wiczer BM, Bernlohr DA . Functional analysis of long-chain acyl-CoA synthetase 1 in 3T3-L1 adipocytes. J Biol Chem 2009; 284: 18347–18356.
Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A . Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009; 50: 3–21.
Chan CB, Harper ME . Uncoupling proteins: role in insulin resistance and insulin insufficiency. Curr Diabetes Rev 2006; 2: 271–283.
Carey AL, Petersen EW, Bruce CR, Southgate RJ, Pilegaard H, Hawley JA et al. Discordant gene expression in skeletal muscle and adipose tissue of patients with type 2 diabetes: effect of interleukin-6 infusion. Diabetologia 2006; 49: 1000–1007.
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100: 8466–8471.
Tsuru M, Katagiri H, Asano T, Yamada T, Ohno S, Ogihara T et al. Role of PKC isoforms in glucose transport in 3T3-L1 adipocytes: insignificance of atypical PKC. Am J Physiol Endocrinol Metab 2002; 283: E338–E345.
Ducluzeau PH, Perretti N, Laville M, Andreelli F, Vega N, Riou JP et al. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes 2001; 50: 1134–1142.
Vogt C, Ardehali H, Iozzo P, Yki-Jarvinen H, Koval J, Maezono K et al. Regulation of hexokinase II expression in human skeletal muscle in vivo. Metabolism 2000; 49: 814–818.
Randle PJ . Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998; 14: 263–283.
Hue L, Taegtmeyer H . The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009; 297: E578–E591.
Hammarstedt A, Jansson PA, Wesslau C, Yang X, Smith U . Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun 2003; 301: 578–582.
Acknowledgements
This work has been carried out as a part of the research program of the Danish Obesity Research Centre (DanORC, www.danorc.dk) and is supported by the Nordic Centre of Excellence (NCoE), programme SYSDIET (www.sysdiet.fi), P No. 070014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pietraszek, A., Gregersen, S., Pedersen, S. et al. Acute effects of monounsaturated fat on postprandial lipemia and gene expression in first-degree relatives of subjects with type 2 diabetes. Eur J Clin Nutr 68, 1022–1028 (2014). https://doi.org/10.1038/ejcn.2014.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.64