Abstract
Aim:
To elucidate the modulation of the chemerin/ChemR23 axis by iptakalim-induced opening of KATP channels and to determine the role of the chemerin/ChemR23 axis in the iptakalim-mediated endothelial protection.
Methods:
Cultured rat aortic endothelial cells (RAECs) were used. Chemerin secretion and ChemR23 protein expression were investigated using Western blot analysis. The gene expression level of ChemR23 was examined with RT-PCR. In addition, the release of nitric oxide (NO) was measured with a nitric oxide assay.
Results:
Homocysteine, uric acid, high glucose, or oxidized low-density lipoprotein (ox-LDL) down-regulated the chemerin secretion and ChemR23 gene/protein expression in RAECs as a function of concentration and time, which was reversed by pretreatment with iptakalim (1-10 μmol/L). Moreover, these effects of iptakalim were abolished in the presence of the KATP channel antagonist glibenclamide (1 μmol/L). Both iptakalim and recombinant chemerin restored the impaired NO production in RAECs induced by uric acid, and the effects were abolished by anti-ChemR23 antibodies.
Conclusion:
Iptakalim via opening KATP channels enhanced the endothelial chemerin/ChemR23 axis and NO production, thus improving endothelial function.
Similar content being viewed by others
Introduction
Endothelial dysfunction, which induces disequilibrium in vascular tension, coagulation, and fibrinolysis, contributes to the pathological progression of cardiovascular diseases. It is a common initial risk factor for cardiovascular diseases, such as hypertension and coronary artery diseases1. Recent clinical trials have demonstrated that endothelial dysfunction is a predictor of the development of cardiovascular events2. Targeting endothelial dysfunction is an important therapeutic strategy for cardiovascular diseases. Iptakalim is a promising endothelial protective drug that opens KATP channels by preferentially activating the SUR2B/Kir6.1 subtype of KATP channels. Iptakalim protects endothelial function by enhancing the endothelial nitric oxide (NO) system3. It inhibits the endothelin-1 (ET-1) system by opening KATP channels, and it prevents the overexpression of adhesive molecules, such as intracellular adhesive molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1)3. Iptakalim also improves endothelial function by the activation of KATP channels, thereby preventing the progression of cardiac hypertrophy to congestive heart failure induced by pressure overload4. Improved endothelial function plays a critical role in the iptakalim-mediated prevention of the pathogenetic progression in cardiovascular diseases. The pathophysiology of endothelial dysfunction is complex and involves multiple mechanisms. However, the mechanism whereby iptakalim activates KATP channels to protect against endothelial dysfunction is not completely understood.
ChemR23, a G protein-coupled receptor, when activated by the fish oil derivative, resolving E1 (RvE1), reduced inflammation by inhibiting the transcriptional activation of NF-κB in neutrophils and by selectively counter-regulating leukocytes and platelets, which may correlate with peripheral atherosclerosis5, 6, 7. Chemerin, the endogenous ligand of ChemR23, is a newly discovered adipokine that regulates adipocyte differentiation and modulates chemotaxis and recruitment of NK cells, dendritic cells, and macrophages8, 9, 10. Chemerin is strongly associated with markers of inflammation, metabolic syndrome components, and atherosclerosis11, for which endothelial dysfunction is a fundamental etiological factor. The chemerin/ChemR23 system also plays an important role in endothelial cells12, 13. ChemR23 is reported to be a multifunctional receptor, but little is known about the relationship between the chemerin/ChemR23 axis and endothelial dysfunction14. We investigated the regulatory mechanisms of the chemerin/ChemR23 axis in dysfunctional endothelial cells induced by homocysteine, uric acid, glucose, and oxidized low-density lipoprotein (ox-LDL) and found that the chemerin/ChemR23 axis restores the impaired NO production and is upregulated by the KATP channel opener, iptakalim, in endothelial cells. Our data suggest that augmentation of the chemerin/ChemR23 axis may be a pivotal mechanism for protecting endothelial function by opening KATP channels.
Materials and methods
Chemicals
Iptakalim was synthesized by Nhwa Thad Pharmaceutical Co Ltd (Xuzhou, China). All other chemicals and materials were obtained from local commercial sources.
Cell culture
Rat aortic endothelial cells (RAECs) were isolated from normal Sprague-Dawley rat aortas as described previously3. The aorta was dissected out, sliced open, and rinsed with phosphate-buffered saline. It was cut into pieces and placed lumen surface-down in 100-mm culture dishes with 6 mL of M199 medium containing 20% fetal bovine serum (FBS) without growth supplements. On the third day, the aorta pieces were rinsed, and the collected cells were cultured in M199 medium containing 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin. The RAECs were grown to confluency at 37 °C in a humidified atmosphere of 95% air and 5% CO2. A cobblestone morphology and positive immunofluorescence with antibodies against von Willebrand factor, factor VII-related antigen, or both confirmed that the cells were endothelial in nature. The culture medium was replaced every other day, and the cells were subcultured until they were approximately 90% to 95% confluent. Cells were seeded in 100-mm culture dishes or 6- or 24-well plates and used after three to five passages.
Experimental treatment
Prior to the experimental procedures, cell growth was arrested by culture in phenol red-free M199 supplemented with 1% FBS and 1% penicillin–streptomycin for 6 h. RAECs were treated with homocysteine at two different concentrations (0.5 or 0.1 mmol/L) for various time periods (6, 32, or 40 h) to determine the effect of iptakalim on chemerin secretion and ChemR23 gene/protein expression. RAECs were pretreated with iptakalim (0.1, 1, or 10 μmol/L) for 8 h before treatment with homocysteine, uric acid, glucose or ox-LDL; 1 μmol/L of glibenclamide was applied 1 h before iptakalim. RAECs were treated with 1 μg/mL of ChemR23 antibody for 1 h, iptakalim and recombinant chemerin for 6 or 8 h, and then with 7.5 mg/L of uric acid for 16 h, to determine NO production.
Western blot analysis
Protein expression of ChemR23
After the treatments, RAECs were lysed with a denaturing lysis buffer, and the protein content of the supernatant was determined. The protein extract was then boiled with sample buffer (Tris 100 mmol/L, pH 6.8, 20% glycerol, 4% sodium dodecyl sulfate (SDS) and 0.2% bromophenol blue) at a protein extract to buffer ratio of 4:1. Protein samples were separated using 8% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to 0.45 μm polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) in a Tris−glycine transfer buffer. The membranes were washed in Tris-buffered saline with Tween 20 (TBS-T, 10 mmol/L Tris, pH 7.5, 0.1 mmol/L NaCl, 1 mmol/L EDTA, 0.1% Tween 20) three times for 5 min, incubated in TBS-T with 5% skimmed milk for 2 h and then incubated overnight at 4 °C with a ChemR23-specific antibody (42 kDa, goat, 1:500, Santa Cruz, CA, USA) and an α-tubulin-specific antibody as a loading control (55 kDa, mouse, 1:500, Santa Cruz) in TBS-T with 5% skimmed milk. The blot was then incubated with a secondary antibody (1:1000, R & D Systems, Abingdon, Oxon, UK) for 2 h. Immunoblots were developed with an ECL Plus substrate (PerkinElmer, LAS GmbH, Rodgau, Germany). The fluorograph film was developed with a Kodak GBX developer after the optimum exposure time and fixed with Kodak GBX (Kodak, Rochester, New York, USA). Protein expression was quantified by scanning the films and analyzing the densitometric volume of bands using a Personal Densitometer and Bio-Rad Quantity One software. The relative protein levels of ChemR23 were normalized to α-tubulin for each lane.
Secretion of chemerin
RAECs were cultured in 6-well plates for Western blot analysis of chemerin secretion. The cell culture supernatant and the total cell lysate were collected after treatment. The cell culture supernatant was mixed with an equal volume of sample buffer and subjected to SDS-PAGE before transfer to polyvinylidene fluoride (PVDF) membranes (0.22 μm). Primary antibodies for chemerin (18 kDa, mouse, 1:2000, Alexis Biochemicals, San Diego, CA, USA) in the cell culture supernatant and the control β-actin (43 kDa, mouse, 1:500, Santa Cruz, CA, USA) in the cell lysates were incubated overnight with the membranes in TBS-T with 5% skimmed milk at 4 °C. Membranes were subsequently washed with TBS-T and incubated with a secondary antibody (1:1000, R & D Systems, Abingdon, Oxon, UK) for 2 h at room temperature. The procedures for immunodetection and development of the fluorograph film were the same as mentioned above. Relative levels of chemerin secretion were normalized to β-actin in the corresponding samples of total cell lysates.
Reverse transcriptase-PCR (RT-PCR)
Total RNA was extracted from RAECs and reverse transcribed to cDNA using reverse transcriptase with random hexamer priming. PCR was performed using primers selected for ChemR23 and β-actin. As a control, β-actin mRNA was amplified from the same RNA preparation. The primer sequences for ChemR23 were as follows: sense, 5′-ACCTATGCCGCTATGGAC-3′; and antisense, 5′-GACAGTGAGCAGGAAGACG-3′, 108 bp. The primer sequences for β-actin were as follows: sense, 5′-GTGTTGTCCCTGTATGCCTCTGG-3′; and antisense, 5′-GCTGTGGTGGTGAAGCTGTAGCC-3′, 197 bp. The PCR was conducted for 35 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 40 s. The final cycle included an extension at 72 °C for 10 min. The PCR products were analyzed on a 2% agarose gel, and the intensity of the bands was measured with densitometry. Results were expressed as the density of ChemR23 normalized to β-actin in the same sample.
NO measurement
To assess the effects of iptakalim and recombinant chemerin on NO release, the cultured RAECs were seeded on 24-well culture plates and grown to full confluence. Because of the instability of NO in physiological solutions, most of the NO is typically rapidly converted to nitrite (NO2−), which is further broken down to nitrate (NO3−). Therefore, the level of the NO2−/NO3− ratio in the culture medium was measured with a Nitric Oxide Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. Briefly, nitrate was converted to nitrite with aspergillus nitrite reductase, and the total nitrite was measured using the colorimetric method of the Griess assay as previously described15.
Statistical analysis
The Student's t-test and one-way ANOVA for paired values were used. Data are expressed as mean ± SD. P<0.05 was considered to be statistically significant.
Results
Chemerin secretion and ChemR23 protein/gene expression are upregulated by the iptakalim-mediated opening of KATP channels in homocysteine-induced dysfunctional RAECs
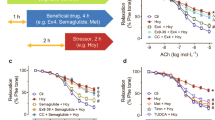
ChemR23 plays an important role in the prevention of cardiovascular events, so we hypothesized that the chemerin/ChemR23 axis was involved in the endothelial protective effect of iptakalim via the opening of KATP channels. We investigated the effect of iptakalim on chemerin secretion (Figure 1A) and ChemR23 protein/gene expression (Figure 1B, 1C) to explore the effects of modulating KATP channel activation on the chemerin/ChemR23 axis in endothelial cells. Homocysteine treatment of RAECs resulted in decreased chemerin secretion and decreased levels of ChemR23 gene/protein expression. A concentration of 0.1–10 μmol/L of iptakalim reversed these decreases in a dose-dependent manner, which was antagonized by the KATP channel antagonist glibenclamide (Figure 1). The opening of KATP channels by iptakalim upregulated chemerin secretion and ChemR23 gene/protein expression in RAECs (Figure 1).
Iptakalim upregulates the chemerin secretion and ChemR23 protein/gene expression in dysfunctional RAECs induced by homocysteine. (A) Representative Western blots and statistical analysis showing the changes in the effect of iptakalim (Ipt) (0.1–10 μmol/L) on chemerin secretion when cells were treated/untreated with 0.5 mmol/L of homocysteine (Hcy) for 40 h. Values are the mean of three separate experiments, and the data represent chemerin normalized to the β-actin. (B) Representative Western blots and statistical analysis showing the changes in the effect of iptakalim (Ipt) (0.1–10 μmol/L) on ChemR23 protein expression when cells were treated/untreated with 0.5 mmol/L of homocysteine for 32 h. Values are the mean of three separate experiments, and the data represent ChemR23 normalized to the α-tubulin. (C) Representative RT-PCR and statistical analysis showing changes in the effect of iptakalim (Ipt) (0.1–10 μmol/L) on ChemR23 gene expression when cells were treated/untreated with 0.1 mmol/L of homocysteine for 6 h (n=4). PCR primers against rat ChemR23 and β-actin amplified a single band of the expected size (108 and 197 bp) in all samples. DNA markers (M) ranging from 100 to 600 bp were used for electrophoresis. Glibenclamide (Glib, 1 μmol/L) was used as a KATP channel antagonist. Data are expressed as mean±SD. The ANOVA test was used. bP<0.05, cP<0.01 vs corresponding control; eP<0.05, fP<0.01 vs homocysteine stimuli;hP<0.05, iP<0.01 vs iptakalim pretreatment, respectively.
ChemR23 gene expression is downregulated by homocysteine, uric acid, glucose, and ox-LDL as a function of concentration and time
Endothelial dysfunction is independently induced by hyperhomocysteinemia, hyperuricemia, hyperglycemia, and hyperlipemia. ChemR23 protein/gene expression was downregulated in homocysteine-induced dysfunctional endothelial cells, so we investigated whether ChemR23 expression was also downregulated by uric acid, glucose, and ox-LDL. RAEC treatment with homocysteine, uric acid, glucose, and ox-LDL resulted in decreased ChemR23 gene expression levels as a function of concentration and time. Increased homocysteine, uric acid, glucose, and ox-LDL levels represent risk factors for cardiovascular diseases associated with endothelial dysfunction. Gene expression levels of ChemR23 are decreased in dysfunctional endothelium (Figure 2).
Downregulation of ChemR23 gene expression as a function of concentration and time by homocysteine (A), uric acid (B), glucose (C), and ox-LDL (D) in RAECs. RAECs were treated for various time periods and various concentrations of homocysteine (Hcy), uric acid, glucose (Glu), and ox-LDL. Data are expressed as mean±SD. n=4. The t-test was used. bP<0.05, cP<0.01 vs the corresponding vehicle control.
Iptakalim opens KATP channels to reverse the downregulation of ChemR23 gene expression induced by uric acid, glucose, and ox-LDL
Iptakalim upregulated the chemerin/ChemR23 axis in homocysteine-induced dysfunctional endothelial cells, so we hypothesized that iptakalim would have the same effect on the chemerin/ChemR23 axis in dysfunctional endothelial cells induced by hyperuricemia, hyperglycemia, and hyperlipemia. A concentration of 0.1–10 μmol/L of iptakalim restored ChemR23 gene expression in a dose-dependent manner in endothelial cells treated with uric acid, glucose, and ox-LDL, and this restoration was antagonized by glibenclamide (Figure 3). Chemerin secretion and ChemR23 protein expression were correlated with ChemR23 gene expression, so they may also be upregulated by iptakalim via the opening of KATP channels when endothelial dysfunction is induced by hyperuricemia, hyperglycemia, and hyperlipemia. Thus, the chemerin/ChemR23 axis may be upregulated by iptakalim via the activation of endothelial KATP channels in dysfunctional endothelial cells.
Iptakalim restores ChemR23 gene expression decreased by uric acid (A), high glucose (B), and ox-LDL (C) in RAECs. RAECs were pretreated with/without iptakalim (Ipt) for 8 h and then stimulated with 7.5 mg/L uric acid for 24 h, 25 mmol/L glucose (Glu) for 16 h, and 100 μg/mL ox-LDL for 12 h. Glibenclamide (Glib, 1 μmol/L) was applied 1 h before iptakalim. Data are expressed as mean±SD. n=4. The ANOVA test was used. bP<0.05, cP<0.01 vs the corresponding control; eP<0.05, fP<0.01 vs uric acid, glucose, or ox-LDL stimuli;iP<0.01 vs iptakalim pretreatment, respectively.
Iptakalim and recombinant chemerin promote NO production, which is involved in the activation of ChemR23 in dysfunctional endothelial cells
A ChemR23 antibody was used to block the effects of iptakalim and recombinant chemerin on endothelial NO production, which helped clarify the role of the chemerin/ChemR23 axis in iptakalim protection against endothelial dysfunction. NO production was decreased when the RAECs were treated with uric acid. Iptakalim (10 μmol/L) upregulated the NO production impaired by uric acid and the effect of iptakalim was blocked by the ChemR23 antibody (Figure 4A). Recombinant chemerin (10, 100, and 300 ng/L) increased NO production compared to uric acid (Figure 4B). The effects of 10 μmol/L of iptakalim and 100 ng/L of chemerin were blocked in the presence of the ChemR23 antibody (1 μg/mL; Figure 4B). Thus, ChemR23 activation directly participates in enhancing the endothelial NO production and is correlated with the opening of KATP channels in endothelial cells.
Iptakalim (A) and chemerin (B) restored NO production decreased by uric acid in RAECs. RAECs were preincubated with/without 1 μg/mL Ab-ChemR23 for 1 h and then treated with iptakalim (Ipt) or recombinant chemerin for 6 or 8 h, respectively, followed by incubation with 7.5 mg/L uric acid for 16 h. NO production in the cell culture supernatant was determined with an nitrate reductase assay at the end of incubation. Data are expressed as mean±SD. n=7. The ANOVA test was used. bP<0.05, cP<0.01 vs corresponding control; eP<0.05, fP<0.01 vs uric acid stimuli; hP<0.05, iP<0.01 vs iptakalim or chemerin pretreatment, respectively.
Discussion
In this study, we showed that iptakalim may regulate the chemerin/ChemR23 axis via the opening of KATP channels in the protection against endothelial dysfunction. Iptakalim restored the impaired chemerin secretion and ChemR23 gene/protein expression induced by homocysteine, uric acid, glucose, and ox-LDL in RAECs. The effects of iptakalim were mediated via KATP channel opening, which was antagonized by glibenclamide. Furthermore, iptakalim and recombinant chemerin restored the NO production that was decreased by uric acid, and this effect was abrogated by anti-ChemR23 antibodies. Our data suggest that the chemerin/ChemR23 axis is a novel molecular pathway that is involved in iptakalim-mediated protection against endothelial dysfunction and cardiovascular pathology.
ChemR23 can be activated by its endogenous ligand chemerin and its exogenous ligand RvE1, which is an endogenous anti-inflammatory mediator derived from eicosapentaenoic acid. ChemR23, when activated by RvE1, induces the phosphorylation of the ribosomal protein S6 of the PI3K-Akt pathway, which may contribute to the resolution of vascular inflammation and ADP-dependent platelet activation in pathological cardiovascular events16, 17. The chemerin/ChemR23 axis may become weaker in pathological conditions, which may not only impair endothelial function, but may also accelerate cardiovascular inflammation18. We demonstrated that the expression of the chemerin/ChemR23 axis fluctuates with endothelial function. We found that homocysteine, uric acid, glucose, and ox-LDL all decreased ChemR23 mRNA levels as a function of concentration and time. Iptakalim protected endothelial function by opening endothelial KATP channels, and it restored the impaired chemerin secretion and ChemR23 gene/protein expression caused by homocysteine, uric acid, glucose, and ox-LDL in endothelial cells. Glibenclamide antagonized the effects of iptakalim, which indicates that the effects of iptakalim are mediated by KATP channel opening. In aortic endothelial cells, KATP channels consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits19. Glibenclamide and iptakalim bind with different sites on the regulatory subunit, the sulfonylurea receptor20. However, some investigators reported that chemerin expression and secretion were enhanced in lupus erythematosus dermal endothelial cells and in interleukin-1β-stimulated adipocytes13, 21. This paradox can be reconciled because these cell types differ in their distinctive physiological functions. ChemR23 expression was downregulated by cardiovascular risk factors, which is consistent with other reports, that showed ChemR23 is downregulated by interleukin-2 or interleukin-15 in NK cells and by proinflammatory cytokines and Toll-like receptor ligands in macrophages9, 22. The downregulation of ChemR23 expression was correlated with the inhibition of cell migration in response to chemerin in NK cells, which may indicate that the receptor function of ChemR23 is decreased along with its downregulation9. Our data suggest that KATP channel opening upregulates the chemerin/ChemR23 axis in dysfunctional endothelial cells, but the mechanism remains unclear.
NO is one of the most important vasodilating substances released by the endothelium, and it inhibits growth and inflammation, has anti-aggregant effects on platelets and plays an important role in cardiovascular diseases. The ability to synthesize NO reflects the functional status of endothelial cells. The endothelial NO system can be downregulated by a number of cardiovascular risk factors, such as hyperhomocysteinemia, hyperuricemia, hyperglycemia, and hyperlipemia3, 15. We detected effects of the chemerin/ChemR23 axis on NO-related endothelial function. We found that iptakalim and chemerin restored NO production; this was decreased by uric acid in RAECs, and this decrease was abolished by anti-ChemR23 antibodies. This suggests that the activation of ChemR23 is necessary for iptakalim and chemerin improvements in endothelial function. Endothelial NO is mainly produced by eNOS, the enzymatic activity of which is regulated at several levels, including Ca2+/calmodulin binding and the interaction of eNOS with associated proteins23. ChemR23 activation resulted in intracellular calcium release promoted by recombinant chemerin in ChemR23-expressing CHO-K1 cells24. Calcium enhanced the phosphorylation of eNOS and NO release by binding to calmodulin25. Although several studies have reported that the chemerin/ChemR23 axis is strongly associated with cardiovascular disease and aspects of metabolic syndrome11, 26, 27, there was no direct evidence for the role of the chemerin/ChemR23 axis in maintaining endothelial function. The chemerin/ChemR23 axis enhances the NO system, so it is likely that the chemerin/ChemR23 axis contributes to the maintenance of endothelial function.
A previous study demonstrated that iptakalim upregulated the NO system and downregulated the endothelin system and the production of adhesion molecules ICAM-1/VCAM-1/MCP-1 in endothelial cells to protect against the progression of cardiac hypertrophy to congestive heart failure3, 4. Endothelial dysfunction is characterized by the reduced bioavailability of NO, an alteration in the production of prostanoids, impairment of endothelium-dependent hyperpolarization, and an increased release of endothelin-1, all of which separately or in association contribute to endothelial dysfunction1. Elevated NO contributes to the equilibrium of vasoactive mediators involved in the regulation of endothelial function and vascular homeostasis. We suggest that the chemerin/ChemR23 axis may attenuate the endothelin system and the overexpression of adhesion molecules in dysfunctional endothelial cells because the chemerin/ChemR23 axis enhances the NO system. Upregulation of the chemerin/ChemR23 axis is a well-supported explanation for how iptakalim improves endothelial function. The chemerin/ChemR23 axis is connected with endothelial function by two lines of evidence. The first is that the chemerin/ChemR23 axis is an endogenous pathway that improves endothelial function. The second is that the chemerin/ChemR23 axis can be modulated by pharmacological intervention via the opening of KATP channels. The chemerin/ChemR23 axis may potentially be a new drug target in endothelial function improvement and cardiovascular disease.
The present study demonstrates that the opening of KATP channels by iptakalim enhances the chemerin/ChemR23 axis in dysfunctional cultured RAECs. ChemR23 activation may be the mechanism by which iptakalim and chemerin increase NO production. This may be a new molecular pathway by which iptakalim upregulates the chemerin/ChemR23 axis to improve endothelial function. A detailed molecular and functional analysis of the role of the chemerin/ChemR23 axis in endothelial function should provide further understanding of the relationship between the chemerin/ChemR23 axis and endothelial function.
Author contribution
Hai WANG designed the study, and Rui-jun ZHAO performed the research, analyzed data, and wrote the paper.
References
Feletou M, Vanhoutte PM . Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol 2006; 291: H985–1002.
Minamino T, Hori M . Protecting endothelial function: A novel therapeutic target of ATP-sensitive potassium channel openers. Cardiovasc Res 2007; 73: 448–9.
Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y . A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res 2007; 73: 497–503.
Gao S, Long CL, Wang RH, Wang H . K(ATP) activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res 2009; 83: 444–56.
Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol 2010; 177: 2116–23.
Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN . Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 2007; 178: 3912–7.
Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008; 112: 848–55.
Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med 2009; 206: 249–58.
Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007; 109: 3625–32.
Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, et al. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol 2009; 297: G1–G10.
Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 2009; 161: 339–44.
Kaur J, Adya R, Tan BK, Chen J, Randeva HS . Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 2010; 391: 1762–8.
Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 2005; 201: 509–15.
Yoshimura T, Oppenheim JJ . Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); Two multifunctional receptors with unusual properties. Exp Cell Res 2011; 317: 674–84.
Long CL, Qin XC, Pan ZY, Chen K, Zhang YF, Cui WY, et al. Activation of ATP-sensitive potassium channels protects vascular endothelial cells from hypertension and renal injury induced by hyperuricemia. J Hypertens 2008; 26: 2326–38.
Fredman G, Van Dyke TE, Serhan CN . Resolvin e1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol 2010; 30: 2005–13.
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN . Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 2010; 285: 3451–61.
Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L . Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 2008; 22: 3595–606.
Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA . KATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol 2004; 37: 857–69.
Pan Z, Huang J, Cui W, Long C, Zhang Y, Wang H . Targeting hypertension with a new adenosine triphosphate-sensitive potassium channel opener iptakalim. J Cardiovasc Pharmacol 2010; 56: 215–28.
Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, et al. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept 2009; 154: 102–6.
Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, et al. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-beta and TLR ligands. Exp Hematol 2006; 34: 1106–14.
Holton M, Mohamed TM, Oceandy D, Wang W, Lamas S, Emerson M, et al. Endothelial nitric oxide synthase activity is inhibited by the plasma membrane calcium ATPase in human endothelial cells. Cardiovasc Res 2010; 87: 440–8.
Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003; 198: 977–85.
Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, et al. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem 2000; 275: 5179–87.
Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH . Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 2010; 17: 115–30.
Ernst MC, Issa M, Goralski KB, Sinal CJ . Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010; 151: 1998–2007.
Acknowledgements
This work was supported by grants from National New Drug Research and Development of Key Project [2009ZX09301-002] and the National 1035 Project 969010101 of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Rj., Wang, H. Chemerin/ChemR23 signaling axis is involved in the endothelial protection by KATP channel opener iptakalim. Acta Pharmacol Sin 32, 573–580 (2011). https://doi.org/10.1038/aps.2011.19
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.19
Keywords
This article is cited by
-
Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis?
BMC Cardiovascular Disorders (2014)
-
A new antihypertensive drug ameliorate insulin resistance
Acta Pharmacologica Sinica (2012)
-
The novel ATP-sensitive potassium channel opener iptakalim prevents insulin resistance associated with hypertension via restoring endothelial function
Acta Pharmacologica Sinica (2011)