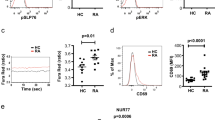
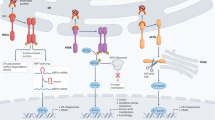
Abstract
Accumulation of fibrils composed of amyloid A in tissues resulting in displacement of normal structures and cellular dysfunction is the characteristic feature of systemic amyloidoses. Here we show that RAGE, a multiligand immunoglobulin superfamily cell surface molecule, is a receptor for the amyloidogenic form of serum amyloid A. Interactions between RAGE and amyloid A induced cellular perturbation. In a mouse model, amyloid A accumulation, evidence of cell stress and expression of RAGE were closely linked. Antagonizing RAGE suppressed cell stress and amyloid deposition in mouse spleens. These data indicate that RAGE is a potential target for inhibiting accumulation of amyloid A and for limiting cellular dysfunction induced by amyloid A.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sipe, J. Amyloidosis Annu. Rev. Biochem. 61, 947– 975, 1992
Falk, R., Comenzo, R. & Skinner, M. The systemic amyloidoses N. Engl. J. Med. 337, 898–908, 1997
Gillmore, J., Hawkins, P. & Pepys, M. Amyloidosis: a review of recent diagnostic and therapeutic developments Br. J. Haematol. 99, 245– 256 (1997).
Hoshii, Y. et al. Amyloid A protein amyloidosis induced in ApoE-deficient mice Am. J. Pathol. 151, 911– 917 (1997).
Wisniewski, T. & Frangione, B. ApoE: a pathological chaperone protein in patients with cerebral and systemic amyloid Neurosci. Lett. 135:235–238 ( 1992).
Kindy, M. & Rader, D. Reduction in amyloid A amyloid formation in apolipoprotein E-deficient mice Am. J. Pathol. 152 , 1387–1395 (1998).
Kindy, M., King, A., Perry, G., deBeer, M. & deBeer, F. Association of apolipoprotein E with murine amyloid A protein amyloid Lab. Invest. 73, 469– 475 (1995).
Hawkins, P., Tennent, G., Woo, P. & Pepys, M. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis Clin. Exp. Immunol. 84, 308–316 (1991).
Botto, M. et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene Nature Med. 3 , 855–859 (1997).
Kisilevsky, R. et al. Arresting amyloidosis in vivo using small molecule anionic sulphonates or sulphates: implications for Alzheimer's disease Nature Med. 1, 143–148 ( 1995).
Rysava, R., Merta, M., Tesar, V., Jirsa, M. & Zima, T. Can serum amyloid A or macrophage colony stimulating factor serve as a marker of amyloid formation? Biochem. Mol. Biol. Int. 47, 845–850 (1999).
Schmidt, A-M., Yan, S-D., Wautier, J-L. & Stern, D. Activation of RAGE: a mechanism for chronic dysfunction in diabetic vasculopathy and atherosclerosis Circ. Res. 84, 489–497 (1999).
Hofmann, M. et al. RAGE mediates a novel proinflammatory axis: the cell surface receptor for S100/calgranulin polypeptides Cell 97, 889–901 (1999).
Yan, S-D., Roher, A., Schmidt, A-M. & Stern D. Cellular cofactors for amyloid beta-peptide induced by cell stress: moving from cell culture to in vivo Am. J. Pathol. 155, 1403 –1411 (1999).
Shiroo, M., Kawahara, E., Nakanishi, I. & Migita, S. Specific deposition of serum amyloid A protein 2 in the mouse Scand. J. Immunol. 332, 721–728 (1998).
Strachen, A., deBeer, F., van der Westhuyzen, D. & Coetzee, G. Identification of three isoform patterns of human serum amyloid A protein Biochem. J. 250, 203–207 (1988).
DeBeer, M., deBeer, F., McCubin, W., Kay, C. & Kindy, M. Structural prerequisites for serum amyloid A fibril formation J. Biol. Chem. 268, 20606–20612 (1993).
Sipe, J. et al. Characterization of the inbred CE/J mouse strain as amyloid resistant Am. J. Pathol. 143, 1480– 1485 (1993).
Park, L. et al. Suppression of accelerated diabetic atherosclerosis by sRAGE Nature Med. 4, 1025–1031 (1998).
Bocchini, V. et al. An immortalized cell line expresses properties of activated microglial cells J. Neurosci. Res. 31, 616 –621 (1992).
Yan, S-D. et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease Nature 382, 685–691 (1996).
Suffredini, A., Fantuzzi, G., Badolato, R., Oppenheim, H. & O'Grady, N. New insights into the biology of the acute phase response J. Clin. Immunol. 19, 203–214 (1999).
Collins, T. Endothelial nuclear factor kB and the initiation of the atherosclerotic lesion Lab. Invest. 68, 499–508 (1993).
Husebekk, A., Skogen, B., Husby, G. & Marbaug, G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo Scan. J. Immunol. 21, 283– 287 (1985).
Kirschner, D., Abraham, C. & Selkoe, D. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibrils in Alzheimer's disease indicates cross-beta conformation Proc. Natl. Acad. Sci. USA 83, 503–507 (1986).
Kimball, M., Sato, A., Richardson, J., Rosen, L. & Low, B. Molecular conformation of erabutoxin b Biochem. Biophys. Res. Comm. 88, 950–956 ( 1979).
Dickson, D. Microglia in Alzheimer's disease and transgenic models Am. J. Pathol. 154, 1627–1631 ( 1999).
Uchihara, T., Akiyama, H., Kondo, H. & Ikeda, K. Activated microglial cells are colocalized with perivascular deposits of amyloid beta-peptide in Alzheimer's disease brain Stroke 28, 1948 –1950 (1997).
Yan, S-D. et al. Amyloid beta peptide-RAGE interaction elicits neuronal expression of M-CSF: a proinflammatory pathway in Alzheimer disease Proc. Natl. Acad. Sci. USA 94, 5296–5301 (1997).
Fixe, P. & Praloran, V. M-CSF: haematopoietic growth factor or inflammatory cytokine? Cytokine 10, 32 –37 (1998).
Hamilton, J. CSF-1 signal transduction J. Leukoc. Biol. 62: 145–155 (1997).
Hume, D. et al. Regulation of CSF-1 receptor expression Mol. Reprod. Dev. 46, 46–52 ( 1997).
Ando, Y. et al. Oxidative stress is found in amyloid deposits in systemic amyloidosis Biochem. Biophys. Res. Commun. 232, 497 –502 (1997).
Ritthaler, U. et al. Expression of RAGE in peripheral occlusive vascular disease Am. J. Pathol. 146, 688– 694 (1995).
Wautier, J-L. et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble RAGE blocks hyperpermeability J. Clin. Invest. 97, 238–243 ( 1996).
Rudderman, N., Williamson, J. & Brownlee, M. Glucose and diabetic vascular disease FASEB J. 6, 2905–2914 ( 1992).
Brett, J. et al. Tissue distribution of RAGE: expression in smooth muscle, cardiac myocytes, and neural tissue in addition to the vasculature Am. J. Pathol. 143, 1699–1712 (1993).
Hori, O. et al. RAGE is a cellular binding site for amphoterin: mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system J. Biol. Chem. 270, 25752 –25761 (1995).
Schafer, B. & Heizmann, C. The S100 family of EF-hand calcium-binding proteins: functions and pathology Trends Biochem. Sci. 21, 134–140 (1996).
Wang, H. et al. HMG1 as a late mediator of endotoxin lethality in mice Science 285, 248–251, 1999.
Taguchi, A. et al. Blockade of RAGE/amphoterin axis suppresses tumor growth and metastases Nature (in the press).
Prelli, F., Pras, M. & Frangione, B. Degradation and deposition of amyloid A fibrils are tissue specific Biochemistry 26, 8251– 8256 (1987).
Klotz, I. & Hunston, D. Mathematical models for ligand-receptor binding J. Biol. Chem. 258, 11442– 11445 (1984).
Acknowledgements
This work was supported by grants from the US Public Health Service (AG00690, AG14103, AG12891, NS31220, HL56881 and HL69091), the Juvenile Diabetes Foundation International and the Surgical Research Fund.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, S., Zhu, H., Zhu, A. et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis . Nat Med 6, 643–651 (2000). https://doi.org/10.1038/76216
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76216
This article is cited by
-
Clinical and Genotype Characteristics and Symptom Migration in Patients With Mixed Phenotype Transthyretin Amyloidosis from the Transthyretin Amyloidosis Outcomes Survey
Cardiology and Therapy (2024)
-
Diagnosis and Treatment of AL Amyloidosis
Drugs (2023)
-
A comprehensive review on RAGE-facilitated pathological pathways connecting Alzheimer’s disease, diabetes mellitus, and cardiovascular diseases
The Egyptian Journal of Internal Medicine (2021)
-
Systemic Amyloidosis: a Contemporary Overview
Clinical Reviews in Allergy & Immunology (2020)
-
The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging
Biogerontology (2019)