Abstract
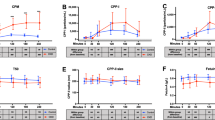
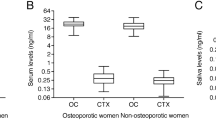
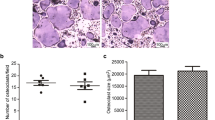
WE have shown previously that a glycoprotein constituent of calcified cortical bone matrix is immunochemically identical to a component of blood plasma1,2. In bone and dentine this specific α-glycoprotein is concentrated in amounts relative to plasma albumin but is not present at greater concentrations than in plasma in various other tissues. This material, although mainly present in extra-vascular sites in bone, could originate from the plasma because it represents a substantial proportion of the radioactivity in bone tissue 12 d after injection of 14C-total plasma glycoprotein1. Rabbit bone α-glycoprotein is analogous to human α2HS-glycoprotein3,4, which is located in mineralising areas in normal human bone matrix5. To our knowledge direct conclusive proof of the tissue origin of the plasma α2HS-glycoprotein is lacking although most of the serum glycoproteins seem to be synthesised by the liver6,7. Nevertheless, its increased concentration in calcified tissues suggests that its presence in plasma could be a result of synthesis and release by bone tissue. We now report that the α2HS-glycoprotein is synthesised by the liver and a proportion is subsequently accumulated in bone tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Triffitt, J. T., and Owen, M. E., Biochem. J., 136, 125–134 (1975).
Ashton, B. A., Triffitt, J. T., and Herring, G. M., Eur. J. Biochem., 45, 525–533 (1974).
Ashton, B. A., Triffitt, J. T., and Höhling, H. J., Calc. Tiss. Res. (in the press).
Schmid, K., and Burgi, W., Biochim. biophys. Acta, 47, 440–450 (1961).
Dickson, I. R., Poole, A. R., and Veis, A., Nature, 256, 430–432 (1975).
Miller, L. L., Hanavan, H. R., Titthasiri, N., and Chowdhury, A., Adv. Chem. Ser., 44, 17–40 (1964).
Miller, L. L., and John, D. W., in Plasma Protein Metabolism (edit. by Rotschild, M. A., and Waldmann, T.), 207–222 (Academic Press, New York and London, 1970).
Hems, R., Ross, B. D., Berry, M. N., and Krebs, H. A., Biochem. J., 101, 284–292 (1966).
Krebs, H. A., and Henseleit, K., Hoppe-Seyler's Z. physiol. Chem., 210, 33 (1932).
Bray, G. A., Analyt. Biochem., 1, 279–285 (1960).
Anker, H. S., in The Plasma Proteins (edit. by Putman, F. W.), II, 267–307 (Academic Press, New York and London, 1960).
Reynolds, J. J., in The Biochemistry and Physiology of Bone, 1, 69–126 (Academic Press, New York and London, 1972).
Herring, G. M., Ashton, B. A., and Chipperfield, A. R., Prep. Biochem., 4, 179–200 (1974).
Blumenthal, N. C., Betts, F., and Posner, A. S., Calc. Tiss. Res., 18, 81–90 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TRIFFITT, J., GEBAUER, U., ASHTON, B. et al. Origin of plasma α2HS-glycoprotein and its accumulation in bone. Nature 262, 226–227 (1976). https://doi.org/10.1038/262226a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/262226a0
This article is cited by
-
Removal of calciprotein particles from the blood using an adsorption column improves prognosis of hemodialysis miniature pigs
Scientific Reports (2023)
-
Serum fetuin-A, tumor necrosis factor alpha and C-reactive protein concentrations in patients with hereditary angioedema with C1-inhibitor deficiency
Orphanet Journal of Rare Diseases (2019)
-
Adipokines in psoriasis: An important link between skin inflammation and metabolic alterations
Reviews in Endocrine and Metabolic Disorders (2016)
-
Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: a double-blind, randomized, controlled trail
European Journal of Nutrition (2015)
-
Identification alpha-2-HS-glycoprotein precursor and tubulin beta chain as serology diagnosis biomarker of colorectal cancer
Diagnostic Pathology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.