Abstract
We aimed to validate prognostic value of elevated admission blood glucose (ABG) for clinical outcomes in diabetic and non-diabetic patients with intracerebral hemorrhage (ICH) in a representative large cohort. Data of ICH patients with onset time ≤24 h were derived from the China National Stroke Registry. Clinical outcomes included 3-month poor outcome (death or dependency) and death. Logistic regression was performed for the association between ABG and clinical outcomes, both in the entire cohort and in patients with and without diabetes mellitus. 2951 ICH patients were enrolled, including 267 (9.0%) diabetics. In the entire cohort, there was a trend to increased risk of poor outcome with increasing ABG levels (adjusted OR 1.09; 95% CI, 1.04–1.15; P < 0.001). The risk of poor outcome was significantly greatest for the highest quartile (≥7.53 mmol/L) of ABG (adjusted OR 1.54; 95% CI, 1.17–2.03; p = 0.002, P for trend 0.004). We got similar association in non-diabetics but not in diabetics. Elevated ABG confers a higher risk of poor outcome in non-diabetics than diabetics with similar glucose level. Elevated ABG is an independent predictor of 3-month poor outcome in ICH patients, the prognostic value of which is greater in non-diabetics than diabetics with similar glucose level.
Similar content being viewed by others
Introduction
Intracerebral hemorrhage (ICH) accounts for 10–15% of all stroke cases in Western countries and up to 20% to 30% in Asian countries1, which is associated with higher rates of death and disability than ischemic stroke2. The evaluation and control of predictors for ICH clinical outcomes is of great importance.
Elevated admission blood glucose (ABG) has been linked to a poor prognosis in patients with ischemic stroke3,4. However, the prognostic value of elevated ABG for ICH outcomes is still under debate and it is unclear whether elevated ABG portended a different prediction based on patients’ diabetic status. Some5,6 but not all7,8 studies have shown that elevated ABG is a predictor of poor outcomes in ICH, most of which are limited to small size, single-centre design, or no direct comparison between diabetics and non-diabetics. Evidences from large-scale multi-centre studies are quite limited9,10, the study subjects of which are limited to specific population such as non-comatose9 or mild to moderate patients10. Accordingly, the purpose of this study is to validate the prognostic value of elevated ABG for clinical outcomes in diabetic and non-diabetic patients with intracerebral hemorrhage (ICH) in a representative large cohort.
Results
Characteristics at baseline
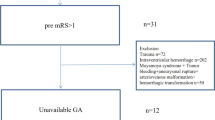
There were 3255 ICH patients meeting the diagnosis criteria for ICH in our study, of which, 288 (8.8%) were excluded due to onset time >24 hours and 16 (0.5%) were excluded for missing ABG measurements (Fig. 1). Among 2951 remaining patients, 1156 (39.2%) were female and 1795 (60.8%) were male. The ages of the participants ranged from 18 to 98 years and mean ± standard deviation were 62 ± 13 years. The median Glasgow Coma Scale (GCS) score of the participants was 14 (interquartile range, 8 to 15), and the median National Institutes of Health Stroke Scale (NIHSS) score was 9 (interquartile range, 3 to 17). The baseline characteristics of the patients are presented in Table 1.
DM was presented in 267 (9.0%) patients. The median (interquartile range) ABG levels for the entire cohort, diabetics and non-diabetics were 6.4 (5.8–7.5) mmol/L, 7.7 (6.3–10.7) mmol/L, and 6.3 (5.7–7.3) mmol/L, respectively. The distributions of ABG levels in these groups are presented in Supplementary Fig. S1. Elevated ABG was common in non-diabetics (e.g., 22% in the highest quartile, 3.6% in the group with ABG ≥11.1 mmol/L). Compared with patients who had lower ABG, patients with elevated ABG were more likely to be female, have more risk factors, had greater stroke severity, were more likely to be with a history of oral hypoglycemic agents and insulin treatment, tended to be treated with neurosurgical intervention, NICU/ICU care or withdraw of support and stayed longer in hospitals.
Associations between ABG and outcomes
No collinearity or significant interactions was found between baseline variables. When ABG concentration was analyzed as a continuous variable, a trend to increased risk of poor outcome with increasing ABG concentration was presented (Fig. 2a) in the entire cohort (adjusted OR 1.09; 95% CI, 1.04–1.15; P < 0.001) and non-diabetics (adjusted Odds ratio [OR] 1.10; 95% confidence interval [CI], 1.04–1.17; P = 0.002), but not in diabetics (adjusted OR 1.03; 95% CI, 0.92–1.16; P = 0.61). Although non-diabetics at the lower ABG levels had a lower poor outcome risk than diabetics, their risk increased more steeply at higher ABG levels, surpassing the risk of diabetics at about 7.0 mmol/L (Fig. 2a, P for interaction <0.001). Similar phenomenon existed for the risk of death, with the risk in non-diabetics surpassing that of the diabetics at an ABG level of 7.5 mmol/L around (Fig. 2b, P for interaction <0.001).
When ABG levels were stratified by quartiles (Table 2), there was a graded increase in the risk of poor outcome as ABG became progressively elevated. The risk of poor outcome was significantly greatest for the highest quartile (adjusted OR 1.54; 95% CI, 1.17–2.03; P = 0.002, P for trend 0.004). Similar results were evident for death (adjusted OR 2.05, 95% CI, 1.44–2.92, P for trend 0.002). These relationships presented in non-diabetics but not in diabetics (Table 3). Similar results were found when reclassifying glucose levels by tertiles or by diagnostic threshold with ABG ≥11.1 mmol/L (Supplementary Table S1 and Supplementary Table S2). Adjusted common OR (95% CI) for quartile 2, quartile 3 and quartile 4 in ordinal regression model were 1.33 (1.09–1.61), 1.32 (1.09–1.61) and 1.47 (1.20–1.79), respectively.
Discussion
It is important to determine whether elevated ABG after ICH is associated with increased poor outcome, since this factor may be therapeutically modified. Our study indicates that elevated ABG is an independent predictor of 3-month poor outcome in ICH patients, the prognostic utility of which might be altered by patients’ diabetic status. Poor outcome risk is greater in non-diabetics with elevated ABG than diabetics with similar ABG level.
Our finding of elevated ABG being a predictor of poor outcomes in ICH is opposite to some previous studies7,8, the small sample size, single-centre design and mixed stroke population of which may account for the discrepancy. Evidence from large-scale multi-centre study9,10 supports the prognosis value of elevated ABG on poor outcomes in ICH. However, being limited to the specific study population, their findings may not be applicable to patients with severe ICH. Meanwhile, the prognostic utility based on patients’ diabetic status is not clearly elucidated in their study due to no direct comparison between diabetics and non-diabetics. Our China National Stroke Registry (CNSR) cohort study demonstrates that the prognostic utility of elevated ABG could be extended to patients with various severity of ICH and indicates that the prognosis utility is caused by a strong association in non-diabetics. The phenomenon that poor outcome risk is greater in non-diabetics than diabetics with similar ABG level after a specific threshold has been found in acute myocardial infarction11. Our study presents this phenomenon in ICH patients as well.
Our study do not shed light on exact pathophysiological mechanisms by which elevated ABG affect prognosis. Previous animal studies indicate that elevated blood glucose may exert deleterious effect on brain through inducing neuronal apoptosis12, increasing superoxide production13, down-regulating the Aquaporin-4 (AQP-4) expression14 and exacerbating perihematomal cell death15 in the brain.
Our data show different prognostic utility of elevated ABG in diabetics and non-diabetics. The possible explanations could be as follows. First, non-diabetics may expose to a greater degree of stress to reach the same hyperglycemic state as diabetic counterparts. Previous study has indicated that stress-induced hyperglycemia conferred higher mortality than diabetic hyperglycemia in trauma16. Second, insulin resistance may exist in non-diabetic patients with elevated ABG17,18. Non-diabetics may expose to a greater degree of fluctuation in insulin resistance to reach the same hyperglycemic state as diabetic counterparts, which confers higher risk of adverse outcome19. Third, some non-diabetics with elevated ABG (particularly those with glucose ≥ 11.1 mmol/L) are undiagnosed diabetics or pre-diabetics and thus may represent a higher-risk cohort. Forth, elevated ABG in non-diabetics was rarely treated during hospitalization11. A better management of elevated ABG in diabetics compared with non-diabetics may result in a relatively better outcome.
Our study validates the associations between elevated ABG and adverse clinical outcomes in ICH patients with various severity and highlight clinical concern on non-diabetic ICH patients with elevated ABG. Whether tight glucose control and different target glucose levels for patients with and without DM can improve the outcome of patients with ICH and elevated ABG needs to be resolved in randomized clinical trials.
Our strengths lie in several aspects. First, our study has a prospective design with representative population and is possibly the largest cohort to date on this issue, which makes our conclusions more reliable. Moreover, associations between ABG and outcomes were compared directly in patients with and without DM to demonstrate different prognostic utility of ABG based on patients’ diabetic status.
Several limitations must be acknowledged. Firstly, the definition of DM status was based only on a medical history at admission, some misclassifications were inevitable. However, the frequency of DM in this study (9.0%) is close to the contemporary prevalence of DM in China20 (9.7% in Chinese adults). Secondly, being limited to the spectrum of glucose values in this study, we failed to examine the association between severer levels of ABG and outcomes in patients with DM.
In conclusion, elevated ABG is an independent predictor of 3-month poor outcome in ICH patients, the prognostic value of which is greater in non-diabetic patients than diabetics with similar glucose level.
Methods
Study Population
The study was based on the CNSR, a nationwide, multi-centre and prospective cohort study, the design, rationale, and baseline information of which has been described in detail elsewhere21. In brief, CNSR was the largest stroke registry of consecutive patients with acute cerebrovascular events between September 2007 and August 2008 in China. 132 hospitals from different regions representing 27 provinces and 4 municipalities in mainland China were selected. CNSR was performed in accordance with the guidelines of the Helsinki Declaration. The protocol and data collection was approved by the Institutional Review Board at Beijing Tiantan Hospital and all participating hospitals. Written informed consent was obtained from each participant or his/her designated relatives.
To be eligible for the diagnosis of ICH in our study, subjects had to meet the following criteria: (1) hospitalized with a primary diagnosis of spontaneous ICH according to the World Health Organization criteria22; (2) not including primary intraventricular ICH, ICH caused by trauma, brain tumor, hemorrhage secondary to malignancy, subarachnoid hemorrhage, arteriovenous malformation and hemorrhagic transformation of cerebral infarct; (3) ICH confirmed by brain CT. CT films were collected and assessed for hematoma volume and evaluation of intraventricular extension. All images were prospectively reviewed by a neuroradiologist from each participating center who was blinded to clinical data. The neuroradiologists of the study centres were trained centrally with the CT protocol23. Patients were excluded if no admission blood glucose data available. Considering variable intervals between symptom onset and ABG measurement, we excluded patients with onset time over 24 hours as well.
Data Collection and Variable Definition
Demographic characteristics, clinical information, radiographic findings, treatment during hospitalization and ABG were collected from the database, as well as hemorrhage evaluation including stroke severity, hematoma volume and hematoma location. Stroke severity was measured using the initial NIHSS score and GCS score. ICH hematoma volumes were measured on the initial brain CT by the ABC/2 method23. Data on hematoma locations, which were classified as supratentorial and infratentorial, and the presence of intraventricular extension were also collected.
ABG was the random blood glucose9,24 measured at the initial emergency department or the blood glucose value from in-hospital immediate evaluation, which was generally done within 3 hours of admission. Patients with a history of diabetes or glucose-lowering treatment before ICH were classified as diabetics, according to previously published articles9,10.
Outcome Measures
The clinical outcomes was poor outcome defined as death or dependency (modified Rankin scale25 [mRS] score of 3 to 6) and death (mRS score of 6) at 3 months, which was assessed by trained study investigators. The telephone follow-up was conducted centrally for all enrolled patients with a standardized interview protocol.
Statistical Analysis
Baseline demographic and clinical characteristics were expressed as mean (standard deviation) or median (interquartile range) for continuous variables and as number (%) for categorical variables. The chi-square test for categorical variables and Mann–Whitney test for continuous variables were used as needed.
Associations of ABG, both as continuous and categorical (quartile) variables, and risk of poor outcome and death were evaluated by separate univariable and multivariable logistic regression. ORs with 95% CIs were calculated. All significant (P < 0.05) baseline variables in the univariable analysis were included in the multivariable analysis. Collinearity and interaction between variables were also check. We further evaluated the associations between ABG and risk of poor outcome and death using a multivariable logistic regression model with restricted cubic splines for ABG adjusting for all confounding factors. The 5 knots for spline were placed at the 5th, 25th, 50th, 75th, 95th percentiles of ABG. These analyses were performed in the entire cohort and repeated in patients with and without DM. Sensitivity analysis including ordinal regression and different categories of ABG (by tertiles and by diagnostic thresholds of blood glucose for DM) were performed to examine the association between ABG and clinical outcomes. A 2-sided P value <0.05 was set as the level for statistical significance. All analyses were performed with SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA).
Additional Information
How to cite this article: Sun, S. et al. Prognostic Value of Admission Blood Glucose in Diabetic and Non-diabetic Patients with Intracerebral Hemorrhage. Sci. Rep. 6, 32342; doi: 10.1038/srep32342 (2016).
References
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9, 167–176 (2010).
Qureshi, A. I., Mendelow, A. D. & Hanley, D. F. Intracerebral haemorrhage. Lancet 373, 1632–1644 (2009).
Nardi, K. et al. Predictive value of admission blood glucose level on short-term mortality in acute cerebral ischemia. J Diabetes Complications 26, 70–76 (2012).
Desilles, J. P. et al. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 44, 1915–1923 (2013).
Fogelholm, R., Murros, K., Rissanen, A. & Avikainen, S. Admission blood glucose and short term survival in primary intracerebral haemorrhage: a population based study. J Neurol Neurosurg Psychiatry 76, 349–353 (2005).
Bejot, Y. et al. The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke 43, 243–245 (2012).
Capes, S. E., Hunt, D., Malmberg, K., Pathak, P. & Gerstein, H. C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32, 2426–2432 (2001).
Tetri, S., Juvela, S., Saloheimo, P., Pyhtinen, J. & Hillbom, M. Hypertension and diabetes as predictors of early death after spontaneous intracerebral hemorrhage. J Neurosurg 110, 411–417 (2009).
Passero, S., Ciacci, G. & Ulivelli, M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology 61, 1351–1356 (2003).
Saxena, A. et al. Prognostic Significance of Hyperglycemia in Acute Intracerebral Hemorrhage: The INTERACT2 Study. Stroke 47, 682–688 (2016).
Kosiborod, M. et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 111, 3078–3086 (2005).
Chiu, C. D. et al. Investigation of the effect of hyperglycemia on intracerebral hemorrhage by proteomic approaches. Proteomics 12, 113–123 (2012).
Won, S. J., Tang, X. N., Suh, S. W., Yenari, M. A. & Swanson, R. A. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol 70, 583–590 (2011).
Chiu, C. D. et al. Hyperglycemia exacerbates intracerebral hemorrhage via the downregulation of aquaporin-4: temporal assessment with magnetic resonance imaging. Stroke 44, 1682–1689 (2013).
Song, E. C. et al. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke 34, 2215–2220 (2003).
Kerby, J. D., Griffin, R. L., MacLennan, P. & Rue, L. W. 3rd Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg 256, 446–452 (2012).
Rundek, T. et al. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch Neurol 67, 1195–1200 (2010).
Kernan, W. N. et al. Insulin resistance and risk for stroke. Neurology 59, 809–815 (2002).
Jotic, A. et al. Decreased Insulin Sensitivity and Impaired Fibrinolytic Activity in Type 2 Diabetes Patients and Nondiabetics with Ischemic Stroke. Int J Endocrinol 2015, 934791 (2015).
Yang, W. et al. Prevalence of diabetes among men and women in China. N Engl J Med 362, 1090–1101 (2010).
Wang, Y. et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke 6, 355–361 (2011).
Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 20, 1407–1431 (1989).
Wang, W. J. et al. Clinical characteristics, management, and functional outcomes in Chinese patients within the first year after intracerebral hemorrhage: analysis from China National Stroke Registry. CNS Neurosci Ther 18, 773–780 (2012).
Zuurbier, S. M. et al. Admission Hyperglycemia and Clinical Outcome in Cerebral Venous Thrombosis. Stroke 47, 390–396 (2016).
Bamford, J. M., Sandercock, P. A., Warlow, C. P. & Slattery, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 20, 828 (1989).
Acknowledgements
This study is supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2006BAI01A11, 2011BAI08B01, 2011BAI08B02, 2012ZX09303-005-001, and 2013BAI09B03).
Author information
Authors and Affiliations
Contributions
S.S. and Y.P. analyzed the data and wrote the manuscript. X.Z., L.L., H.Y. and H.L. performed the research. Y.L.W., Y.J.W. and L.G. conceived, designed and supervised the study. All authors reviewed and finally approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, S., Pan, Y., Zhao, X. et al. Prognostic Value of Admission Blood Glucose in Diabetic and Non-diabetic Patients with Intracerebral Hemorrhage. Sci Rep 6, 32342 (2016). https://doi.org/10.1038/srep32342
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32342
This article is cited by
-
Pre-stroke glycemic variability estimated by glycated albumin predicts hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage
Scientific Reports (2023)
-
Association of early glycemic change with short-term mortality in lobar and non-lobar intracerebral hemorrhage
Scientific Reports (2021)
-
The frequency and impact of admission hyperglycemia on short term outcome of acute stroke patients admitted to Tikur Anbessa Specialized hospital, Addis Ababa, Ethiopia: a cross-sectional study
BMC Neurology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.