Abstract
Recent evidence regarding mechanical chest compressions in out-of-hospital cardiac arrest (OHCA) is conflicting. The objective of this study was to perform a meta-analysis of randomized controlled trials (RCTs) to compare the effect of mechanical versus manual chest compressions on resuscitation outcomes in OHCA. PubMed, Embase, the Cochrane Central Register of Controlled Trials and the ClinicalTrials.gov registry were searched. In total, five RCTs with 12,510 participants were included. Compared with manual chest compressions, mechanical chest compressions did not significantly improve survival with good neurological outcome to hospital discharge (relative risks (RR) 0.80, 95% CI 0.61–1.04, P = 0.10; I2 = 65%), return of spontaneous circulation (RR 1.02, 95% CI 0.95–1.09, P = 0.59; I2 = 0%), or long-term (≥6 months) survival (RR 0.96, 95% CI 0.79–1.16, P = 0.65; I2 = 16%). In addition, mechanical chest compressions were associated with worse survival to hospital admission (RR 0.94, 95% CI 0.89–1.00, P = 0.04; I2 = 0%) and to hospital discharge (RR 0.88, 95% CI 0.78–0.99, P = 0.03; I2 = 0%). Based on the current evidence, widespread use of mechanical devices for chest compressions in OHCA cannot be recommended.
Similar content being viewed by others
Introduction
Out-of-hospital cardiac arrest (OHCA) claims hundreds of thousands of lives annually all over the world. Only 1% to 8% of victims of OHCA survive to hospital discharge1 and up to half of survivors have some level of brain damage that may or may not completely resolve2. In the early cardiopulmonary resuscitation (CPR), high-quality chest compressions (i.e., sufficient depth, rate, full recoil of the chest and avoidance of interruptions) are crucial to both cardiac and brain resuscitation3,4.
Mechanical compression devices have been developed to provide high-quality chest compressions without the interruptions and fatigue associated with manual chest compressions. At present, there are two main mechanical compression devices: a load-distributing band CPR (LDB-CPR) device that provides circumferential thoracic compressions and a piston-driven CPR (PD-CPR) device that provides sternal compressions. Preclinical and observational studies suggest that mechanical chest compressions may increase cerebral blood flow, coronary perfusion pressures and cardiac output to improve survival compared with manual chest compressions5,6.
So far, application of mechanical compression devices during CPR has been evaluated in several systematic reviews. In the settings of out-of-hospital and in-hospital cardiac arrest, a Cochrane systematic review suggested insufficient evidence on the effectiveness of mechanical versus manual chest compressions7. Recently, a meta-analysis of randomized and observational studies focusing on OHCA showed a significant improvement in return of spontaneous circulation (ROSC) rate with mechanical chest compressions but did not assess survival outcomes8. Since then, several large randomized controlled trials (RCTs) addressing this topic have been published with conflicting results9,10,11. Given the limitations of observational studies with respect to managing risk of bias, in order to provide the latest and solid evidence, we conducted a meta-analysis of RCTs comparing the effect of mechanical versus manual chest compressions on survival and neurological outcomes in participants with OHCA.
Materials and Methods
Ethical approval and patient consent were not required since this was a meta-analysis of previously published studies. The present meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement12.
Literature search and selection criteria
Relevant articles were identified by searching PubMed, Embase, the Cochrane Central Register of Controlled Trials and the ClinicalTrials.gov registry (up to March 18, 2015). Electronic searches were conducted using Exploded Medical Subject Headings and the appropriate corresponding keywords, including “Mechanical chest compression”, “load distributing band”, “piston driven compression”, “AutoPulse” and “Lucas”. No language restriction was imposed. Additionally, the reference lists of the original studies and previous review articles were hand-searched to identify other potentially eligible studies.
Two authors (Tang L and Gu WJ) independently assessed the eligibility of all studies identified in initial research. Studies meeting the following criteria were included: (1) population: adult participants with non-traumatic OHCA; (2) intervention: mechanical chest compressions; (3) comparison: manual chest compressions and (4) design: RCTs. Agreement regarding trial inclusion was assessed using the Cohen К statistic13.
Data extraction and outcomes definition
Two authors (Tang L and Gu WJ) independently extracted the following data: first author, year of publication, study location, participant characteristics, mechanical compression device, resuscitation strategy, adverse events and main outcomes using a standard form. The original authors were contacted if data needed clarification or were not presented in the publication. Any disagreement was resolved by discussion.
The primary outcome was survival with good neurological outcome to hospital discharge. Secondary outcomes included survival to hospital admission, survival to hospital discharge, ROSC and long-term (≥6 months) survival. Neurological outcome was assessed using the Cerebral Performance Category (CPC) score or the modified Rankin Scale (mRS) score. Good neurological outcome was defined as the CPC score ≤ 214 or the mRS score ≤ 315. Survival to hospital admission was defined as patient admission to the hospital without ongoing CPR or other artificial circulatory support. ROSC was defined as the presence of any palpable pulse, in the absence of chest compression and detectable by manual palpation. When data on survival to hospital admission was not available, we extract data on 4-hour survival after successful ROSC. In case that survival to hospital discharge was not reported, data on survival to 30 days were extracted. With respect to long-term survival, data on survival to six months or more were extracted.
Risk of bias and evidence grade assessment
Two authors (Tang L and Gu WJ) independently assessed risk of bias in included RCTs with the method recommended by the Cochrane Collaboration16. Disagreements were resolved by consensus. The quality of evidence for the outcome measures was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach17. A summary table was prepared using the GRADE profiler (GRADEpro, version 3.6).
Statistical analysis
All analyses were on an intention-to-treat basis. Differences were presented as relative risks (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. The Mantel-Haenszel method with the random-effects model was used to calculate pooled RRs and 95% CIs. Heterogeneity was quantified using the I2 statistic and if the I2 value was greater than 50%, heterogeneity was considered to be substantial. A priori subgroup analysis was conducted according to mechanical compression devices (LDB-CPR and PD-CPR). Publication bias was assessed by visually inspecting a funnel plot. P < 0.05 was considered statistically significant. All statistical analyses were conducted using Review Manager software (version 5.3; Nordic Cochrane Centre, Cochrane Collaboration).
Results
Study identification
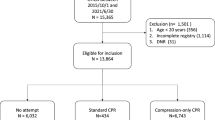
The comprehensive search yielded 648 citations, of which 633 were eliminated for various reasons based on the title and abstract. The full texts of the remaining 15 publications were scrutinized for further evaluation. Finally, five RCTs9,10,11,18,19 with a total of 12,510 participants were included (Fig. 1). The Cohen statistic К for agreement on study inclusion was 0.93.
Study characteristics
The main characteristics of the included RCTs are presented in Table 1. The trials were published between 2006 and 2015 and were all multicenter studies. The sample size ranged from 148 to 4471. Among the included trials, two10,18 compared LDB-CPR with manual CPR and the remaining three compared PD-CPR with manual CPR. Four9,10,18,19 included trials were sponsored by the manufacturer of the mechanical compression devices and the remaining one trial11 was funded by National Institute for Health Research. Age, cardiac etiology, witnessing of cardiac arrest, bystander CPR and presenting rhythm were generally similar between the trial groups.
Risk of bias assessment
Randomized sequence was adequately generated in two trials10,11 and allocation sequence concealment was adequately reported in four trials9,10,11,19. Although blinding of participants and personnel and blinding of outcome assessors were difficult to implement due to the nature of the intervention, survival and neurological outcome assessment were very unlikely to have been influenced by knowledge of trial allocations. Thus, the two items (blinding of participants and personnel and blinding of outcome assessors) were judged to be low risk of bias for all the included trials. The numbers and reasons for withdrawal/dropout were detailed reported in all trials. One trial18 was defined as having other sources of bias because it was stopped early after the interim analysis. An overview of the risk of bias is summarized in Fig. 2.
Primary outcome
Data on the primary outcome was available in four of the included RCTs (n = 12,058). Mechanical chest compressions did not significantly improve survival with good neurological outcome to hospital discharge (RR 0.80, 95% CI 0.61–1.04, P = 0.10; I2 = 65%; Fig. 3) compared with manual chest compressions. Subgroup analyses stratified by mechanical compression devices also suggested no significant difference in the primary outcome both in the LDB-CPR subgroup and in the PD-CPR subgroup.
Secondary outcomes
Compared with manual chest compressions, mechanical chest compressions were associated with worse survival to hospital admission (RR 0.94, 95% CI 0.89–1.00, P = 0.04, I2 = 0%; Fig. 4) and to hospital discharge (RR 0.88, 95% CI 0.78–0.99, P = 0.03; I2 = 0%; Fig. 5). Subgroup analyses suggested worse survival to hospital admission and to hospital discharge were indicated in the LDB-CPR subgroup but not in the PD-CPR subgroup.
No significant differences were observed in ROSC (RR 1.02, 95% CI 0.95–1.09; P = 0.59; I2 = 0%; Fig. 6) or long-term (≥6 months) survival (RR 0.96, 95% CI 0.79–1.16; P = 0.65; I2 = 16%; Fig. 6) between the mechanical CPR group and the manual CPR group.
Quality of evidence and publication bias
The GRADE evidence profiles for the primary and secondary outcomes were shown in Table 2. The quality of evidence was low for survival with good neurological outcome to hospital discharge; moderate for survival to hospital admission, survival to hospital discharge, ROSC and long-term (≥6 months) survival.
For publication bias, the funnel plot was not conducted due to the small number of RCTs included.
Discussion
The accumulated evidence from meta-analysis of RCTs suggested that mechanical chest compressions did not improve survival with good neurological outcome to hospital discharge, ROSC or long-term (≥6 months) survival and were associated with worse survival to hospital admission and to hospital discharge in OHCA compared with manual chest compressions.
Differences between the current meta-analysis and a previous one by Westfall et al.8 should be noted. First, Westfall et al.8 conducted a meta-analysis of English-language randomized and non-randomized (phased, historical and case-control) studies. Thereinto, only 2 small studies were RCTs18,19 and most of the included studies were non-randomized, thereby producing a potential for selection bias. Our meta-analysis included only RCTs and three large RCTs9,10,11 published since 2014 were included, which substantially enlarged the sample size (12,510 vs. 6,538 participants). Second, considering that survival data are deemed to be one of the highest quality and/or most clinically relevant endpoints for a given trial, we chose survival with good neurological outcome to hospital discharge, a more patient-centered outcome measure, as the primary outcome. Additionally, survival to hospital admission, survival to hospital discharge, ROSC and long-term (≥6 months) survival were also reported in the present meta-analysis. However, in Westfall et al.’s meta-analysis8, the primary outcome was ROSC and survival data were not reported. Third, the methods used for risk of bias assessment and GRADE profile evidence were described in our meta-analysis not in Westfall et al.’s8. Last, in Westfall et al.’s meta-analysis8, all authors have financial relationships with a manufacturer of mechanical compression devices, while in our meta-analysis, none of the authors have financial relationships with the manufacturers.
In the present meta-analysis, the findings of worse survival to hospital admission and to hospital discharge in participants receiving mechanical chest compressions were unexpected. One possible explanation is that interruptions in CPR during device deployment could cause reduced cardiac and cerebral perfusion. Furthermore, device deployment before the first defibrillation is likely to have led to a delay in the time to first defibrillation, which might in itself reduce survival. In one included RCT18, the time to first defibrillation was delivered 2.1 minutes later in the LDB-CPR group than in the manual CPR group. Similarly, in one included RCT evaluating the PD-CPR device9, the mechanical compression group had an average 1.5-minute extra delay to first defibrillation attempt. Therefore, if mechanical chest compression devices are being used in OHCA, special attention should be paid to minimizing the delay to chest compressions and defibrillation related to deployment of the device.
Since interruptions in CPR and delays in device deployment are a major factor that can impact outcomes, intensive and repetitive training is particularly important when using the mechanical compression devices in OHCA. In Perkins et al.’s study11, only 60% participants in the PD-CPR group received the allocated intervention and about 15% cases of non-use were because of difficulties inherent with implementation of new equipment and the training and quality issues associated with this. In a recent study20, mechanical chest compressions were associated with a higher no-flow ratio than manual chest compressions in the first five minutes of resuscitation. Where organizations decide to adopt mechanical compression device, it seems essential that sufficient resources are made available to support initial and regular refresher training and ongoing quality assurance.
Clinical adverse events occurring in chest compressions were reported in three included RCTs9,10,11, such as rib fractures, pulmonary edema, pneumothorax and chest bruising. Specifically, the type of device and the mechanism of the device were thought to be of paramount relevance to injury patterns observed in OHCA. A non-randomized study reported that the PD-CPR device slid from its original position, mostly in an abdominal direction during transport21. Although pooled analyses were not conducted due to significant clinical heterogeneity, no significant difference in serious adverse events was reported in individual included RCT.
The quality of evidence in the present meta-analysis ranked from moderate to low across the different outcomes. The limiting factor that was the reason for a decrease in quality for all outcomes was the serious risk of bias. Only two of the included RCTs were judged to be at low risk of bias. Additionally, due to the unexplained heterogeneity (I2 = 65%) across the small number of included studies, the evidence quality for the survival with good neurological outcome to hospital discharge was downgraded by one more level and was therefore assessed as low quality.
There are several limitations in the present study. First, although comprehensive literature search was conducted, the number of included RCTs was small and the funnel plot for publication bias was not conducted. Second, although more and more services emphasize the importance of ongoing performance monitoring for out-of-hospital CPR quality, none of the included RCTs reported any measures of CPR quality and we cannot assess the influence of the quality of manual CPR on the treatment effect estimates. Future studies comparing mechanical with manual CPR should include the instrumentation to monitor CPR quality. Third, although subgroup analyses suggested that LDB-CPR not PD-CPR significantly decreased survival to hospital admission and to hospital discharge than manual CPR, caution should be taken when interpreting the results because they were based on the pooled analysis of only two RCTs.
Conclusions
Our meta-analysis suggested that mechanical chest compressions were not associated with better outcomes including survival with good neurological outcome to hospital discharge, survival to hospital admission, survival to hospital discharge, ROSC and long-term (≥6 months) survival in OHCA compared with manual chest compressions. Based on the current evidence, widespread use of mechanical devices for chest compressions in OHCA cannot be recommended.
Additional Information
How to cite this article: Tang, L. et al. Mechanical versus manual chest compressions for out-of-hospital cardiac arrest: a meta-analysis of randomized controlled trials. Sci. Rep. 5, 15635; doi: 10.1038/srep15635 (2015).
References
Lombardi, G., Gallagher, J. & Gennis, P. Outcome of out-of-hospital cardiac arrest in New York City. The pre-hospital arrest survival evaluation (phase) study. JAMA 271, 678–83 (1994).
Young, G. Neurologic prognosis after cardiac arrest. New England Journal of Medicine 361, 605–11 (2009).
Ewy, G. A. Cardiocerebral resuscitation: The new cardiopulmonary resuscitation. Circulation 111, 2134–42 (2005).
Stiell, I. et al. Health-related quality of life is better for cardiac arrest survivors who received citizen cardiopulmonary resuscitation. Circulation 108, 1939–44 (2003).
Rubertsson, S. & Karlsten, R. Increased cortical cerebral blood flow with lucas; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation 65, 357–63 (2005).
Steen, S., Liao, Q., Pierre, L., Paskevicius, A. & Sjoberg, T. Evaluation of lucas, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation 55, 285–99 (2002).
Brooks, S. C., Hassan, N., Bigham, B. L. & Morrison, L. J. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database of Systematic Reviews Issue 2. Art. No.: CD007260. doi: 10.1002/14651858.CD007260.pub3 (2014).
Westfall, M., Krantz, S., Mullin, C. & Kaufman, C. Mechanical versus manual chest compressions in out-of-hospital cardiac arrest: A meta-analysis. Crit Care Med. 41, 1782–9 (2013).
Rubertsson, S. et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: The linc randomized trial. JAMA 31, 53–61 (2014).
Wik, L. et al. Manual vs. Integrated automatic load-distributing band cpr with equal survival after out of hospital cardiac arrest. The randomized circ trial. Resuscitation 85, 741–8 (2014).
Perkins, G. D. et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (paramedic): A pragmatic, cluster randomised controlled trial. Lancet 385, 947–55 (2015).
Liberati, A. et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700 (2009).
Sim, J. & Wright, C. C. The kappa statistic in reliability studies: Use, interpretation and sample size requirements. Phys Ther. 85, 257–68 (2005).
Jennett, B. & Bond, M. Assessment of outcome after severe brain damage. Lancet 1, 480–4 (1975).
Rittenberger, J. C., Raina, K., Holm, M. B., Kim, Y. J. & Callaway, C. W. Association between cerebral performance category, modified rankin scale and discharge disposition after cardiac arrest. Resuscitation 82, 1036–40 (2011).
Higgins, J. P. T. (editors) GS. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011]. The cochrane collaboration. (2011) Available from www.Cochrane-handbook.Org.
Guyatt, G. H. et al. Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–6 (2008).
Hallstrom, A. et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: A randomized trial. JAMA 295, 2620–8 (2006).
Smekal, D., Johansson, J., Huzevka, T. & Rubertsson, S. A pilot study of mechanical chest compressions with the lucas device in cardiopulmonary resuscitation. Resuscitation 82, 702–6 (2011).
Ong, M. E. et al. Cardiopulmonary resuscitation interruptions with use of a load-distributing band device during emergency department cardiac arrest. Ann Emerg Med. 56, 233–41 (2010).
Axelsson, C., Nestin, J., Svensson, L., Axelsson, A. B. & Herlitz, J. Clinical consequences of the introduction of mechanical chest compression in the ems system for treatment of out-of-hospital cardiac arrest-a pilot study. Resuscitation. 71, 47–55 (2006).
Acknowledgements
The study was supported by research grant for outstanding young scientists from Shandong Province, China (No. BS2014SW010), grant from the National Natural Science Foundation of China (No. 81501650) and the research projects from General Hospital of Jinan Military Command, China (No. 2013BS01, 2013BS09).
Author information
Authors and Affiliations
Contributions
T.L. and G.W.-J. contributed substantially to the definition of inclusion and exclusion criteria, electronic and manual search of the literature, drafting and revision of the manuscript, study design and analysis and interpretation of the data. F.W. contributed to the study design, interpretation of the results and writing, revision and approval of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tang, L., Gu, WJ. & Wang, F. Mechanical versus manual chest compressions for out-of-hospital cardiac arrest: a meta-analysis of randomized controlled trials. Sci Rep 5, 15635 (2015). https://doi.org/10.1038/srep15635
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15635
This article is cited by
-
Interventions to improve cardiopulmonary resuscitation: a review of meta-analyses and future agenda
Critical Care (2019)
-
Neue Leitlinien zur kardiopulmonalen Reanimation und ihre Implikationen für die herzchirurgische Intensivmedizin
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.