Abstract
Characterisation of protective helminth acquired immunity in humans or experimental models has focused on effector responses with little work conducted on memory responses. Here we show for the first time, that human helminth infection is associated with altered proportions of the CD4+ memory T cells, with an associated alteration of TH1 responses. The reduced CD4+ memory T cell proportions are associated with a significantly lower ratio of schistosome-specific IgE/IgG4 (marker for resistance to infection/re-infection) in uninfected older people. Helminth infection does not affect the CD8+ memory T cell pool. Furthermore, we show for the first time in a helminth infection that the CD4+ memory T cell proportions decline following curative anti-helminthic treatment despite increased CD4+ memory cell replication. Reduced accumulation of the CD4+ memory T cells in schistosome-infected people has implications for the development of natural or vaccine induced schistosome-specific protective immunity as well as for unrelated pathogens.
Similar content being viewed by others
Introduction
The adaptive immune system, largely orchestrated by lymphocytes, is central to the development of acquired immunity against current and subsequent infection with pathogens. T lymphocytes are key regulators and effectors of the adaptive immune responses. Upon contact with specific antigen (through natural infection or vaccination), they differentiate and expand into two populations, effector and memory cells. The generation and persistence of the latter provides the basis for an efficient immune response in subsequent encounters with the pathogen preventing or reducing re-infection.
CD4+ T cells are central in the development of protection against re-infection with human helminth parasites including schistosomes (see review1). To date, helminth vaccine development has focused on inducing CD4+ effector responses directed against the parasites with little understanding of the dynamics of CD4+ memory responses2,3,4. Compared to CD8+ memory relatively less is known about the development of CD4+ memory T cells during human infections. Furthermore, even less is known about the development of CD4+ memory during chronic antigen stimulation from parasites as occurs in the presence of schistosome eggs trapped in the liver, or during repeated re-infection events as occurs in populations endemically exposed to helminth infections. These features of helminth infections are likely to influence the development of naturally acquired immunity as well as the efficacy and immunopathological consequences of helminth vaccines, for example vaccinating people already exposed to the parasite may result in pathology as reported from a trial of a human hookworm vaccine candidate5. Understanding the interaction between helminth infection and the overall host immune responses is important for optimising vaccination against schistosomes as well as unrelated parasites. There is a growing body of literature indicating that helminths can modulate the adaptive immune responses directed against themselves as well as immune responses directed against unrelated, so called bystander antigens6,7. Furthermore, descriptive studies in humans have shown that vaccine efficacy is reduced in helminth infected individuals a phenomenon that has largely been attributed to the development of regulatory responses (reviewed in8), but may also be related to failure to optimally develop memory responses. To date, there have been few studies on the interaction between helminth parasites and the development of memory T cell responses in people exposed to/infected with helminth parasites. Recently a study in a small group of 29 people exposed to the nematode parasite Wuchereria bancrofti, which causes lympatic filariasis, suggested that alteration of memory T cell responses may be involved in the immunomodulation of memory T cell responses in individuals with patent infection9.
Therefore, we set out to determine whether infection with a helminth parasite is associated with changes in the memory T cell pool in humans and also determine to what extent the function of effector cells would be altered. The study focused on people naturally exposed to the parasite Schistosoma haematobium, commonly known as the blood fluke. Populations resident in areas endemic for schistosomiasis show a characteristic age-infection profile with infection intensity rising early during childhood, peaking around 9–14 years and then declining in adulthood10 a pattern largely attributed to the development of acquired immunity as a result of cumulative exposure to parasite antigens11,12.
The processes and drivers of the generation, differentiation and persistence of memory T lymphocytes in humans are less well characterised relative to the mouse model13. Nonetheless, several key features of human memory T lymphocytes have been described. CD4+ memory and CD8+ memory T cell accumulate with host age relative to naïve T cells14,15 due to reduced thymic output of naïve T cells and accumulation of memory T cells in response to constant exposure to pathogenic and environmental antigens16. CD8+ memory cell differentiation and homeostasis is relatively well understood17,18, whereas the mechanisms of CD4+ memory T cell generation and persistence are still being debated13,19,20. Since the mechanisms of CD4+ memory T cell generation are less well described, it is not predictable whether helminths are potentially able to modulate this generation. Therefore, the first aim of this study was to determine if the age-related accumulation of memory T cells differs in people infected with helminths compared to uninfected people. The second aim of the study was to determine the effects of curative anti-helminthic treatment on the memory T cell pool, since curative anti-helmintic treatment results in both increased reactivity against helminth antigens and possible improved vaccine efficacy in helminth endemic areas8,21,22. Mechanistic studies of how anti-helminthic treatment may mediate this remain unexplored and may include alterations in T cell memory proportions.
Results
Helminth epidemiology in study population
Since this study focused on S. haematobium an area with low prevalences of S. mansoni and soil-transmitted helminths (STH) was selected for the study based on previous National Schistosomiasis surveys23 and pre-surveys showing a low prevalence of S. mansoni (<2%) and the absence of STH. Only lifelong residents and thus people exposed to schistosomiasis throughout their life by frequent contact to infective water as assessed by questionnaire (allowing age to be used as a proxy for their cumulative history of exposure to schistosomiasis)24, but who had never received anti-helminthic treatment were enrolled in the study. Therefore, egg negative young children are yet to be infected while egg negative old people have developed resistance to infection/re-infection. All participants were negative for HIV and Plasmodium infection.
105 participants (schistosome infection prevalence = 61.0% and mean infection intensity = 38.9 eggs per 10 ml urine (SEM = 8.5, range 0–571.0) were enrolled in the study. Partitioning the participants by age as is routine24,25, showed that infection levels follow the typical age-infection profile for schistosome infections in high transmission areas of rising and peaking in childhood and declining rapidly thereafter26. In this population infection levels are high in the three youngest age groups (aged 6–17 years) (details in Table 1 and Supplementary Figure 1), but decline significantly in people aged 18 and above.
CD4+ but not CD8+ memory T cells differ between infected and uninfected older individuals
Proportions of total CD4+ or CD8+ T cells within CD3+ T cells or lymphocytes did not differed between uninfected and infected people in any of the four age groups (Supplementary Figure 2).
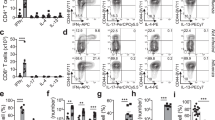
The CD3+ T cell compartment was divided into CD4+ and CD8+ T cells and naïve and memory T cells in these subsets were differentiated using CD45RA as a marker and quantified. As depicted in Figure 1a, there were significantly higher proportions of CD4+ memory T cells in uninfected compared to infected individuals. No significant differences were observed in CD8+ memory T cell proportions (Figure 1b). Further statistical analysis showed that age, but not sex had a significant influence on CD4+ memory T cell proportions whereas CD8+ memory T cells were not affected by either of these variables (Table 2).
CD4+ and CD8+ memory T cells in S. haematobium infections.
PBMC were gated on CD3+CD4+ (a) or CD3+CD8+ (b) positive cells and analysed for the proportions of memory cells by gating on CD45RA- cells. Proportions are expressed as percentages of total CD4+ or CD8+ cells respectively. Data were subsequently grouped in uninfected and infected people. Means are shown as gray line and ranked data are compared by a non-parametric Mann-Whitney-U test. CD3+CD4+CD45RA- T cells were further divided into CD3+CD45RA-CD62L- TEM (c) and CD3+CD45RA-CD62L+ TCM (d) and compared between uninfected and infected individuals. Means are shown as gray line and compared by a non-parametric Mann-Whitney-U test.
Low CD4+ memory T cell proportions are associated with current infection
In order to determine if the differences in CD4+ memory T cells was due to current infection or the history of infection, serological levels of whole worm homogenate (WWH)-specific IgM, an indicator of current exposure to parasite antigens and IgE, an indicator of memory responses to parasite antigens were compared between the schistosome-infected and uninfected participants. The ratio of IgM to IgE as an indicator of the relative contribution was negatively correlated to CD4+ memory T cell (r = −0.246, p = 0.032) meaning that lower CD4+ memory T cells are associated with higher worm-specific IgM and/or lower IgE and are thus associated with current rather than previous infection.
Furthermore it could be that individuals with lower proportions of CD4+ memory T cells are more likely to be infected and therefore low CD4+ memory T cells are the cause rather than the consequence of an infection. To address this hypothesis we selected individuals who were not infected at baseline. Infection levels of the same individuals were again assessed 18 month later to determine if those individuals were infected within this period. Subsequently proportions of CD45RA-CD4+ T cells were compared between people who remained uninfected within this period and became infected (Supplementary Figure 3). We did not observe difference in CD4+ memory T cell proportion between those two groups indicating that low levels of CD4+ memory T cells are correlated to higher susceptibility to infection.
The rate of which CD4+ memory T cell accumulate with age differs between schistosome infected and uninfected individuals
The differences in CD4+ T cell proportions in schistosome infected versus uninfected people differed with host age (Table 2). To further dissect how the relationship between CD4+ memory T cells and infection status varied with age, the data were divided into four age groups and uninfected were compared to infected individuals (Figure 2a). Whereas in the two younger age groups the proportions of CD4+ memory T cells are similar between uninfected and infected individuals, in the third age group (13–17 years) there are significantly lower CD4+ memory T cells. In the oldest age group (18+ years), there are fewer CD4+ memory T cells in infected versus uninfected people but this difference was not statistically significant. This pattern was not observed for CD8+ memory T cells (Figure 2b).
Proportions of CD4+ and CD8+ memory T cells in different age groups.
CD3+CD4+CD45RA- (a), CD3+CD8+CD45RA- (b), CD3+CD45RA-CD62L- TEM (c) and CD3+CD45RA-CD62L+ TCM (d) populations were further divided into four age groups. Means ± SEM are presented and infected (dashed lines) versus uninfected (solid lines) were compared. Differences between infected and uninfected were tested by ANOVA.
To determine the dynamics of the age-related changes in CD4+ memory T cell proportions, data were divided by infection status and the correlation coefficients between CD4+ T cell proportions and age (continuous across age groups) compared (Table 3). In both uninfected and infected individuals CD4+ memory T cells rose significantly with age, but the rate of this increase was higher in the uninfected group compared to the infected group with significant differences in the correlation coefficients as assessed by analysis of homogeneity (Table 3).
To gain insight in potential mechanism responsible for altered proportions of CD4+ memory T cells expression levels of IL-7Rα on CD45RA- memory T cells were analysed. As indicated in Supplementary Figure 4. No differences between uninfected and infected individuals in any of the four age groups was observed. In addition we analysed expression levels of the inhibitory receptor CTLA-4/CD152. These data were only available on a subset of samples (N = 51, aged 6–17 years). As shown in Figure 3a in infected individuals proportions of CTLA-4+CD4+ T cells significantly increased with age (β = 0.473, p = 0.005). This correlation with age was not observed in uninfected people (β = 0.119, p = 0.662). Proportions of CTLA-4+ CD4+ T cells differ between uninfected and infected people in the age groups ranging from 10–17 years.
Uninfected older individuals have increased WWH-specific IgE/IgG4 ratios indicating the development of resistance and show differences in their CTLA-4+CD4+ T cells.
The proportions of CTLA-4+CD4+ T cells (a) and the ratio of whole worm homogenate WWH-specific IgE/IgG4 (b) were determined, data divided into age groups and the difference between uninfected (solid lines) and infected (dashed lines) was analysed. Data are derived from a subset of the total cohort (N = 51, aged range 5–17 years), of which those data are available.
The rate of development of both the TEM and TCM CD4+ memory T cell compartments differs between schistosome infected versus uninfected individuals
CD4+CD45RA- memory T cells were further subdivided in central memory (TCM) and effector memory (TEM) subpopulations based on their expression of the CD62 Ligand (CD62L) with TCM being CD62L+ and TEM being CD62L-. TEM cells accounted for the majority of CD4+ memory T cells (mean of 71.8%±1.4% CD62L-CD45RA- of CD45RA-CD4+ T cells) and showed a similar pattern to total CD4+ memory T cells. Schistosome-infected individuals showed lower TEM than uninfected people (Figure 1c) and after partitioning the population into age groups, TEMcell proportions followed the same profile (Figure 2c). A correlation analysis between age and proportions of CD4+ memory T cells was performed (Table 3). TEM increases with age in both infected and uninfected individuals, but the correlation coefficient differs significantly between both infected and uninfected groups indicating a delayed development of TEM. In addition a subset of samples has been analysed for TEM and TCM using CCR7, an alternative marker to differentiate between CD4+ TEM and TCM. Proportions of TEM defined by either CCR7 or CD62L correlate to each other (p = 0.001). The age-infection the age-infection pattern obtained CD45RA-CCR7- or CD62L- CD4+ memory T cells are comparable.
The proportions of TCM do not significantly differ between infected and uninfected people (Figure 1d). Correlation analysis revealed that there is a positive association between age and TCM in uninfected individuals, but none in infected individuals. In infected individuals TCM rather peak at the age of 10–12 years decline again and subsequently increase in the oldest age group (Figure 2d).
Curative anti-helminthic treatment results in a reduction of CD4+ memory T cell proportion
In a next step we assessed if treatment with the anti-helmintic drug praziquantel affects the proportions of CD4+ memory T cells. This would be particularly informative since the analysis above indicated that current exposure to schistosome infection as measured by IgM was associated with lower levels of CD4+ memory T cells. Proportions of CD4+ memory T cells were compared pre- and six weeks post-treatment. Following inclusion criteria detailed in the methods section, a total of 50 people were enrolled in the cohort study with 36 treated people and 14 untreated people who formed the control group. As indicated in Figure 4a no significant change was observed in the proportions of total CD4+ memory T cells in untreated individuals between the two time points. However in treated individuals the proportions of CD4+ memory T cells decreased significantly (Figure 4b) (p = 0.015). No significant changes occurred in CD8+ memory T cell proportions in either of the treated or untreated groups (Figure 4c, d).
Effect of anti-helminthic treatment on CD4+ and CD8+ memory T cells.
CD3+CD4+CD45RA- memory T cells (a, b) and CD3+CD8+CD45RA- memory T cells (c, d) were quantified in either untreated controls (a, c) (N = 14) or in individuals who received praziquantel (b, d) (N = 36) at baseline and six weeks later. P – values obtained from a Wilcoxon paired sample test.
The post-treatment decrease in CD4+ memory T cell proportions is associated with increased proliferation of CD4+ memory T cells
To determine possible reasons for the decrease in CD4+ memory T cells in treated people, we analysed the proportions of CD31+CD4+ T cells. CD31+CD4+ T cells have been recently shown to be a marker of recent thymic emigrants and CD31 is mainly expressed on naïve CD45RA+CD4+ T cells27,28. Therefore an increase of CD31+CD4+ T cells would indicate changes in thymic output of naïve CD4+ T cells. However, as indicated in Figure 5a and b, there was no significant change pre- and six weeks post treatment in proportions of CD31+CD4+ T cells. Next we analysed if there was a change in the replicative history of CD4+ memory T cells by analysis of their telomere length. CD4+CD45RO+ memory T cells were purified using magnetic beads and subsequently genomic DNA was isolated. Finally the relative length of telomeres was analysed by quantitative PCR. We found an increase in telomere length on CD4+ memory T cells in the untreated group indicating newly generated CD4+ memory T cells within the six week period (Figure 5c).
Effect of anti-helminthic treatment on CD31+CD4+ T cells and telomere length of CD4+ memory T cells.
CD4+CD31+ T cells were quantified in PBMC samples either obtained from untreated controls (a) or individuals treated with praziquantel (b) at baseline and six weeks later. CD4+CD45RO+ memory T cells were isolated from PBMC obtained from either untreated controls (N = 11) (c) or praziquantel treated people (N = 15) (d) using magnetic beads. DNA was isolated and telomere length quantified using qPCR. Presented data are normalized to a single copy gene (36b4).
In contrast, in treated individuals telomere length was significantly reduced in CD4+ memory T cells six weeks after treatment (Figure 5d) suggesting that those CD4+ memory T cells underwent more proliferation cycles following treatment.
The S. haematobium mean infection intensity in analyzed untreated people increased from 77.5 (SEM = 18.7) to 111.0 eggs/10 ml urine (SEM = 47.0), whereas the infection intensity of treated people decreased from 100.2 (SEM = 39.3) to 0.023 eggs/10 ml urine with only one individual having infection not cleared. We therefore hypothesized that the changes in telomere length might be related to changes in the infection intensity in a combined analysis of both treated and untreated individuals. As shown in Supplementary Figure 5 the difference in infection intensity and the difference in telomere length were significantly correlated to each other.
Functional impairment of CD4+ memory T cells
Next the relationship between the number of CD4+ memory T cells and their potential to produce IFN-γ (as marker of TH1 effector function) in a non-specific manner was analysed. PBMC were stimulated with the mitogen phytohaemagglutinin PHA for 48 hours and supernatants analysed for the production of cytokines. IFN-γ production was significantly associated with CD4+ memory T cells proportions in uninfected individuals unlike in infected individuals where there was no significant correlation between the proportion of CD4+ memory T cell proportions and IFN-γ production (Table 4). In contrast, neither of the two TH2 associated cytokines (IL-4 and IL-5) correlated with the proportions of CD4+ memory T cell in infected or infected individuals after statistically controlling for the effects of sex and age. The activation status of effector/memory CD4+ T cells was assessed by determining the proportion of HLA-DR+CD4+ T cells. In uninfected individuals CD4+ memory T cells were positively associated with HLA-DR+CD4+ cells after allowing for the effects of sex and age (Table 4). In contrast, there was no significant association between CD4+ memory T cells and HLA-DR+CD4+ T cells in infected people.
Schistosome-specific responses in uninfected versus infected people
To analyse if parasite specific cellular cytokine responses are altered in people infected with S. haematobium we analysed cytokine production by WWH-stimulated PBMC. The TH2 cytokine IL-4 did not differ between infected and uninfected and infected individuals in any of the analysed age groups (data not shown). Parasite-specific IFN-γ was slightly higher in uninfected individuals (mean 15.4 pg/ml ± SEM 3.9) compared to uninfected (mean 11.8 pg/ml ± SEM 2.7) with the difference being most pronounced in the age group of 10–12 years. However the interaction between age group and infection status was statistically not significant. In addition none of these parasite specific cytokines show a correlation to proportions of CD4+ memory T cells.
In addition to the cytokine responses, we also measured levels of the schistosome-specific IgE and IgG4 directed against the adult stage of the parasite which have been experimentally shown to be associated with resistance and susceptibility to infection respectively29. We used the ratio of IgE/IgG4, an accepted marker of resistance to infection/re-infection30,31,32, to analyze the schistosome-specific memory response. In younger individual no difference in the WWH-specific IgE/IgG4 ratio was observed in infected versus uninfected people (Figure 3b). However, older uninfected individuals (who have been exposed to schistosome infections earlier in life) had a higher WWH-specific IgE to IgG4 ratio (F2,49 = 6.043, p = 0.018) consistent with the development of resistance to schistosome infection.
Discussion
Helminth infections are chronic in nature providing constant antigenic stimulation for prolonged periods of time. In addition people resident in helminth endemic areas experience multiple infection events before the expression of protective acquired immunity. The effect of these two features on the nature, development and maintenance of memory responses remains largely unknown. These basic characteristics of helminth immune responses influence the development of protective acquired immunity making their understanding a critical step in harnessing the development of much needed vaccines against human helminth infections4. Furthermore, gradually accumulating evidence suggests that helminth infections can reduce the efficacy of vaccines against unrelated pathogens through immunological mechanisms warranting an urgent understanding of the development of both effector and memory responses against schistosomes and other pathogens in people exposed to persistent and repeated helminth infections. During persistent antigenic stimulation, down-regulation of effector T-cell mediated33,34 and antibody responses35 as a result of immune exhaustion has been described. However, these studies have focused on CD8+ T cells with relatively less work conducted on CD4+ T cells35,36. An experimental study in Plasmodium has suggested that in contrast to the results in models for CD8+ T cells, continuous exposure to parasite antigens is required for the maintenance of CD4+ T cell-mediated immunological protection against the parasite37. Our studies and those of others have shown that acquired immunity associated with protection against schistosome infection requires a threshold of antigen to develop and that the slow development of acquired resistance to schistosome infection reflects the time it takes to accumulate this antigen38. Moreover, repeated exposures to parasite antigens following anti-helminthic treatment result in the development of resistance against re-infection39. Yet how these different features of helminth infection affect the development of the T cell memory compartment of the immune system has not been previously studied in human populations.
To our knowledge, this makes this present study the first cohort study which documents the dynamics of effector/memory T cell proportions in a population exposed to a helminth infection. Our study focused on people who had been exposed to schistosome infection throughout their lives so that their age was a proxy for their cummulative history of schistosome infection. Overall the proportion of CD4+ memory T cells was significantly higher in uninfected people compared to infected people. The uninfected group constitutes a heterogenous group in terms of their history of schistososme infection. Uninfected young people represent people who have yet to be infected, while uninfected older people represent people who have cleared infection encounted earlier in life and are currently resistant to re-infection despite continued exposure to infective water24. The study showed that independent of schistosome infection, the proportion of CD4+ and CD8+ memory T cells increased with age as has been reported for other human populations14,15. However, the proportion of CD4+ memory T cells but not CD8+ memory T cells differed significantly in people currently infected with schistosomes compared to uninfected people, resulting in delayed accumulation of CD4+ memory T cells in the schistosome infected people. Despite starting at similar levels, the increase of CD4+ memory T cell populations with age in infected people is delayed compared to uninfected people. Protective acquired immunity against schistosomes develops with age and the rate at which this occurs is determined by the intensity of infection12,24. The age group with the highest proportion of memory CD4+ T cells are uninfected adults who also had the highest levels of schistosome specific IgE which is associated with resistance to re-infection. CD4+ memory T cells have been shown to mediate protection against re-infection in experiemental helminth infection40 and the age-related pattern in CD4+ memory T cells in this population suggests that this population of cells is involved in the development of protective immunity against the parasites.
To assess the relative contribution of a current versus a previous infection to the proportions of CD4+ memory T cells, the levels of adult worm-specific IgM and IgE were determined. IgM reflects levels of current exposure to parsite antigens while IgE reflects the development of protective immunity which develops following cummulative exposure to parasite antigens giving an indication of previous exposure to parasite antigens. Thus, the ratio between the two provides a measure or current versus previous exposure to parasite antigens. The negative association between the worm-specific IgM/IgE ratio and the CD4+ memory T cell proportions suggests that current infection rather than history of infection is associated with reduced CD4+ memory T cell proportions.
In this population, TEM proportions were higher than TCM which is contrary to published data for European donors41, but comparable to the proportions reported in Malawians and Brazilians42,43 both countries where schistosome infections are endemic44. The proportions of TEM cells which mediate migration to peripheral tissue was significantly lower in schistosome infected versus uninfected people. This was not the case for TCM cells. TEM express a repertoire of receptors which mediate migration to peripheral tissue. In older individuals chronic schistosome infection is often associated with severe immuno-pathology45 caused by effector/memory CD4+ T cells. Therefore, it is possible that the lower levels of TEM cells in schistosome infected people might be due to enhanced migration to the site of infection. However, in our study, the CD4+ T cell memory pool is altered rather than only the parasite-specific memory T cells. That would mean that the migration would mean that the migration capacity of CD4+ memory T cells with different specifities might be altered even under steady state conditions without other present infections. This possibility needs further evaluation.
Furthermore we analyzed the expression of the inhibitory receptor CTLA-4/CD152 which can limit proliferation of T cells46 and been suggested to play a role in experimental filarial infection47. We could show that the proportions of CTLA-4+ T cells are higher in the age groups from 10–17 years of age providing a potential mechanism of limiting CD4+ memory T cells over time. The difference in CTLA-4+ T cells was already observed in the 10–12 years old individuals suggesting that it needs some time until the inhibitory effect of CTLA-4 to be reflected in CD4+ memory T cells proportions. Although CTLA-4 is expressed on regultory T cells it has also been suggested that CTLA-4 plays crucial role on CD4+Foxp3- T cells47.
In uninfected people, both TCM and TEMproportions increase with host age. In schistosome infected people, TEM also increase with age albeit at lower levels than in uninfected people, but the TCMpeaked in the age group of 10–12 years. The TEM cell pool may be reflecting the development of protective immune responses, while the TCM pool may be reflecting exposure to the parasite antigens as has been reported for IgE and IgM responses against schistosomiasis24,39. Thus, it seems the combination of lower TEM and different TCM-age profile results in the differences observed in the CD4+ memory T cell pool in schistosome infected versus uninfected participants in the two older age groups. This contrasts with the results reported from a study in filarial-infected patients, which showed a reduced TCM compartment, but tended to have more TEM9. Possible explanations for the discrepancy between the two studies are differences in antigen concentrations in the blood and differences related to the parasites, since it has been suggested that antigen load can influence TEM and TCM proportions48,49 and immune responses between nematodes and trematodes differ1.
One possible explanation for the differences in the proportions and dynamics of CD4+ memory T cells in schistosome-infected versus uninfected participants may be the differences in antigen presentation. CD4+ effector and memory T cells require more substantial presentation of antigens by antigen presenting cells than CD8+ memory T cells50. In this population, we observed age-related differences in the proportion of plasmacytoid and myeloid DCs in schistosome-infected versus uninfected people (Nausch et al submitted) which is consistent with results published by other groups where schistosome-infected people had reduced levels of HLA-DR, leading to reduced T cells activation51. Another reason might be a regulation of IL-7Rα on CD4+ memory T cells, however levels of IL-7Rα did not differ in our study.
Curative anti-helminthic treatment of schistosome infected people results in both the reversal of schistosome-related immuno-modulation52 and improved efficacy of vaccines as shown for a malaria vaccine in an experimental model53, but mechanistic explanations for these observations have yet to be fully explained. In this study curative anti-helminthic treatment resulted in a significant decline in the proportion of CD4+ memory T cells which was not observed in untreated indviduals. There was no increased output of naïve T cells in these treated individuals as determined by proportions of CD31+CD4+ T cells27,28. Therefore, a possible explanation for the decrease in the CD4+ memory T cells post-treatment is that the increased proliferation of these cells (indicated by reduced telemore lengths) following the increased antigenic stimulation from antigens released by dying worms/removal of immunosuppression, is subsequently followed by self limitation54 and therefore reduced proportions of CD4+ memory T cells. This activation may not be limited to schistosome-specific CD4+ memory T cells, but may also lead to an activation/proliferation of CD4+ memory T cells with different specifities to other antigens55. Such an increase of non-specific proliferation following treatment has been reported before56. The fact that the telomere length in treated people did not increased indicates that reactivation of already exisiting, but inhibited CD4+ memory T cells contribute to an enhanced proliferation and contradicts the hypothesis that newly generated CD4+ effector/memory T cells contribute to it.
The telomere length of the CD4+ memory T cell pool increased in untreated people. Increased telomere length in CD4+ memory T cells might indicate enhanced generation of memory T cells from naïve cells. We could show that changes in infection intensity were associated with changes in telomere length. Therefore increased telomere length in CD4+ memory T cells in untreated individuals might be associated with the observed increase in S. haematobium infection intensity.
Changes in infection intensity would correlate whith changes in levels of antigens present. A recent experimenatal study has shown that chronic/repeated exposure is required to maintain effective CD4+ effector/memory T cells in malaria and treatment changes their proportions37.
The study also showed that the functional activity of the CD4+ memory T cells measured as the potential of the cells to produce IFN-γ was impaired in schistosome infected individuals compared to uninfected individuals an effect which was not observed for TH2 cytokines. The strong modulation of activated CD4+ memory T cells was supported by the fact that HLA-DR+CD4+ were positively associated with CD4+ memory T cells in uninfected people, but not in infected individuals.
The functional relevance of the higher CD4+ memory T cells as well as mechanism involved in the expression of protective immunity against schistosome infection still require investigation. In this study we demonstrated that the ratio of antibody responses shown to mediate protection and susceptibility to infection (IgE and IgG4 respectively) which is a marker for resistance against infection i.e. the IgE/IgG4 ratio, was significantly higher in egg negative older people coinciding with the highest levels of CD4+ memory T cells. However, similar to previous studies by both ourselves and others, there was no significant relationship between schistosome-specific cytokine responses and infection status.
Taken together this study shows that infection with the helminth parasite S. haematobium is associated with a non-specific modulation of the CD4+ effector/memory T cell pool but not CD8+ cell pool. This results in a delay in the accumulation of CD4+ memory T cells and is accompanied with alteration of TH1 type responses. The mechanisms of this modulation remain to be investigated, but changes in antigen-presenting cell populations present a possible explanation. Anti-helmintic treatment results in changes in the proliferation of the CD4+ memory T cells and an associated decline in the proportion of CD4+ memory T cells. These results are important for improving our understanding of the development of helminth-specific responses with age, knowledge which can be harnessed for the development of vaccines against these important human and veterinary parasites. Furthermore, since these effects are not restricted to schistosome-specific responses, the reduced memory T cell populations in schistosome infected people have the potential to influence the efficacy of unrelated vaccines and the aetiology/progression of immune disorders that are mediated by CD4+ responses. Further mechanistic studies in experimental models complemented by human studies will clarify the role of memory T cells in the development of protective immunity against helminth infections as well as the development of responses to non-parasite (bystander) antigens.
Methods
Ethical statement
Permission to conduct the study in the region was obtained from the Provincial Medical Director and institutional and ethical approval was received from the University of Zimbabwe's Ethical Review Board and the Medical Research Council of Zimbabwe respectively. Only compliant participants were recruited into the study and they were free to drop out at any point during the study. At the beginning of the study, participants and their parents/guardians (in case of children) had the aims and procedures of the project explained fully in the local language, Shona and written consent and assent were obtained from participants and parents/guardian before parasitology and blood samples were obtained. After collection of all samples, all participants were offered anti-helminthic treatment with the recommended dose of praziquantel (40 mg/kg of body weight).
Study population and parasitology
This study was performed in two villages, Magaya and Chipinda, which are located in the Mashonaland East Province of Zimbabwe (31°91'E; 17°63'S). The villages were selected because health surveys regularly conducted in the region showed little or no infection with other helminths, a low S. mansoni prevalence (<2%) and high S. haematobium prevalence as defined by World Health Organisation on the criteria of having an infection prevalence greater that 50%57. Villagers are subsistence farmers who have frequent contact with infective water (as assessed by questionnaires) due to insufficient safe water and sanitation facilities as is typical in rural Zimbabwe58. Drinking water is collected from open wells while bathing and washing is conducted in perennial rivers surrounding the village. This area has not been included in any schistosome control programmes and therefore participants had not received anti-helminthic treatment for schistosomiasis or other helminth infections meaning that their natural immune responses could be studied in the absence of drug-altered schistosome responses11. Urine and stool samples were collected on three consecutive days and analysed for S. haematobium (urine filtration) and S. mansoni and intestinal helminths (Kato-Katz method) respectively using standard procedures59,60.
Participants were screened for malaria infection (Plasmodium falciparum) by microscopic examination of Giemsa stained blood smears and results confirmed using a serological rapid test (Paracheck-PF®, Orchid Biomedical Systems, Goa, India). HIV status was determined by immunochromatography (DoubleCheckGold™ HIV 1&2, Orgenics) with HIV positive samples subsequently re-tested by a second rapid assay (Determine HIV 1/2 Ag/Ab Combo, InvernessMedical) to confirm HIV status. For inclusion into the study participants had to meet the following criteria (1) be life-long residents in this area (assessed by questionnaires), (2) should not have received anti-helminthic treatment prior this study, (3) should have provided at least two urine and two stool samples and (4) should have tested negative for intestinal helminths as well as for S. mansoni (5) and should have tested negative for HIV and Plasmodium infection and (6) should have provided sufficient blood for subsequent analysis. The study area is mesoendemic for Plasmodium infection61 and none of the participants were positive for malaria. To relate immune responses to schistosome infections alone, all participants were selected to be negative for HIV infection. These exclusion criteria did not bias the population in terms of their schistosome epidemiology since the prevalence of HIV in the whole study population was low at 8%.
The selected cohort comprised 105 participants (43 males, 62 females) with an age range from 6–84 years (mean 16.2, details in Table 1). For some analyses (presented in Figure 3) only a subset of this cohort could be analysed due to limited amount of sample material. These analyses included 51 participants aged 6–17 years. After collection of all samples, all participants were offered treatment with the recommended dose of praziquantel (40 mg/kg of body weight).
To determine the effects of anti-helminthic treatment on the memory T cells, participants were followed up after treatment. Six weeks following treatment, participants provided urine, stool and blood samples as described above. This period of six weeks was chosen to allow a high cure rate in terms of egg output62, but to avoid establishment of patent re-infections. 36 treated participants were selected, who have provided stool and urine samples (as described above) as well as sufficient blood before and after treatment. In addition 14 individuals which either missed or refused treatment (for religious reasons), but still were willing to be part of the study and fulfilled listed the criteria formed the untreated control group. The age of the group ranged from 7–17 years (mean 12.6 years). Participants who had missed treatment were offered treatment with the standard dose of praziquantel after sample collection.
Blood collection and isolation of PBMC
Depending on participant age up to 25 ml of venous blood was collected in heparinised tubes of which approximately 5 ml was used for serological assays as well as microscopic detection of malaria parasites. The remaining blood was used for the isolation of peripheral blood mononuclear cells (PBMC) through density gradient centrifugation using Lymphoprep™ (Axis-Shield, Cambridgeshire, UK). These PBMC were subsequently enumerated, cryo-preserved and stored in liquid nitrogen in Zimbabwe prior to shipping to Edinburgh in dry shippers for assaying.
Phenotyping of PBMC
Thawing of cryo-preserved PBMC was performed by rotating cryovials in a 37°C water bath until a small crystal was remaining in the cell suspension. Cells were then slowly re-suspended in RPMI 1640 supplemented with 10% FCS, 2 mM L-glutamine and 100 U Penicillin/Streptomycin (all Lonza, Verviers, Belgium). Cells were washed twice with the corresponding media, counted and viability (mean 82.2 % ± SEM = 1.7%) assessed using trypan blue (Sigma-Aldrich, Dorsert, UK). Cryo-preserved PBMCs have been previously used to assess changes CD4+ memory T cells and have been stored for much longer than in our study9. In addition a comparison between fresh and cryo-preserved did not show difference in naïve or memory T cell proportions. Cells were washed with Dulbecco's-PBS (Lonza) and surface stained with the following antibodies: PerCP-conjugated anti-CD3 (OKT-3), Alexa488-conjugated anti-CD4 (clone OKT-4), e450-conjugated anti-CD8 (RPA-T8), PE-conjugated anti-CD45RA (HI100), PE-Cy7-conjugated anti-CD62L (DREG-56; all eBioscience, San Jose, USA), Pacific blue-conjugated anti-CD31 (WM59, Biolegend) and PE-Cy7-conjugated anti-HLA-DR (L243, BD Bioscience). Stained cells were acquired on a FACSCantoII™ (BD Biosciences) and analyzed using FlowJo software software (TreeStar, USA).
Cytokine and antibody assays
For the detection of cytokines PBMC (0.25x106) were stimulated with 1 μg/ml phytohemagglutinin (PHA, Sigma) or with 10 μg/ml WWH in X-vivo™ 15 medium (Lonzo, Verviers, Belgium) for 48 hours. Supernatants were harvested and analysed for the cytokines IL-4, IL-5, IFN-γ, using BD CBA array Flex set bead technology following the manufacture's instructions and a BD FACSarray™ Bioanalyzer was used to acquire data (BD Bioscience).
Enzyme-linked immunosorbent assays (ELISA) for adult-worm specific IgM, IgG4 and IgE were performed as described previously63,64.
Determination of Telomere length
CD45RO+ CD4+ memory T cells were isolated using magnetic beads following instructions of the manufacturer (Miltenyi Biotec, Germany). CD4+ T cells were isolated using a negative selection kit (Miltenyi Biotec, 130-091-155) followed by a positive selection using CD45RO microbeads (130-048-001). Cells were washed, counted and genomic DNA was isolated using Qiamp DNA blood mini kit (Qiagen, Germany). Quantitative PCR followed a previously reported protocol65. For telomere amplification the following primers were used: 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′ and 5′-GGCTTGCCTTACCCT TACCCTTACCCTTACCCTTACCCT-3′ both at a concentration of 200 nM. The single copy gene 36b4 was used as control with following primers: 5′- CAGCAAGTGGGAAGGTGTAATCC-3′ and 5′- CCCATTCTATCATCAACGGGTACAA-3′ both at 300 nM. SYBR-green PCR was carried out using a Precision™ master mix kit (PrimerDesign). PCR was conducted in duplicate and performed using a LightCycler® 480 II (Roche). The enzyme was activated at 95°C for 10 min following 40 cycles of 10 sec at 95°C, 10 sec at 56°C for telomeres and 10 sec at 58°C for 36b4 followed by 60 sec at 60°C (telomere) or 50 sec at 60°C (36b4). Ct values for telomeres were normalized using the single copy gene 36b4.
Statistical analysis
All statistical tests were conducted using the software package SPSS using methods described by Mutapi and Roddam66. P values were taken to be significant at p < 0.05. In an initial analysis it was determined if there were overall significant differences in the proportions of memory T cells between schistosome infected and uninfected participants, a Mann-Whitney U test was conducted. To further analyse which factors influence the proportions of T cell subsets or WWH-specific antibody (dependent variable) transformed data (T cell proportions were arcsine square root transformed as is standard for transforming proportions into continuous data67, whereas antibody levels were square root transformed) were used (to allow the use of parametric tests) in univariate analysis of variance using sex (male/female), host age group (age groups 1–4) and infection status (uninfected = 0 mean egg count per 10 ml and infected > 0 mean egg count per 10 ml; all categorical) as independent variables and contrast analysis between infected and uninfected individuals was performed within each age group.
To determine the relationship between CD4+ memory T cells and cytokine data and between CD4+HLA-DR+ T cells and CD4+ memory T cells, a partial correlation controlling for the effects of sex and age was used. For this analysis, cytokine data were square root transformed whereas CD4+ T cell subsets were arcsine square root transformed.
To address the question of how anti-helminthic treatment affected memory T cell proportions, a repeated measurement ANOVA was used.
References
Bourke, C. D., Maizels, R. M. & Mutapi, F. Acquired immune heterogeneity and its sources in human helminth infection. Parasitology 138, 139–159 (2011).
Capron, A., Riveau, G., Capron, M. & Trottein, F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol 21, 143–149 (2005).
McManus, D. P. & Loukas, A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev 21, 225–242 (2008).
Hotez, P. J. & Brown, A. S. Neglected tropical disease vaccines. Biologicals 37, 160–164 (2009).
Bethony, J. M. et al. Vaccines to combat the neglected tropical diseases. Immunol Rev 239, 237–270 (2011).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-{beta} pathway. J Exp Med 207, 2331–2341 (2010).
Harnett, W. & Harnett, M. M. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol 10, 278–284 (2010).
Labeaud, A. D., Malhotra, I., King, M. J., King, C. L. & King, C. H. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis 3, e442 (2009).
Steel, C. & Nutman, T. B. Altered T cell memory and effector cell development in chronic lymphatic filarial infection that is independent of persistent parasite antigen. PLoS One 6, e19197 (2011).
Woolhouse, M. E. J. Immunoepidemiology of intestinal helminths: pattern and process. Parasitology Today 8, 192 (1992).
Mutapi, F. et al. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis 178, 289–293 (1998).
Woolhouse, M. E. J., Taylor, P., Matanhire, D. & Chandiwana, S. K. Acquired immunity and epidemiology of Schistosoma haematobium. Nature 351, 757–759 (1991).
Lees, J. R. & Farber, D. L. Generation, persistence and plasticity of CD4 T-cell memories. Immunology 130, 463–470 (2010).
Saule, P. et al. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev 127, 274–281 (2006).
Xu, X., Beckman, I., Ahern, M. & Bradley, J. A comprehensive analysis of peripheral blood lymphocytes in healthy aged humans by flow cytometry. Immunol Cell Biol 71 ( Pt 6), 549–557 (1993).
Naylor, K. et al. The influence of age on T cell generation and TCR diversity. J Immunol 174, 7446–7452 (2005).
Rocha, B. & Tanchot, C. CD8 T cell memory. Semin Immunol 16, 305–314 (2004).
Wherry, E. J. & Ahmed, R. Memory CD8 T-cell differentiation during viral infection. J Virol 78, 5535–5545 (2004).
Seddon, B., Tomlinson, P. & Zamoyska, R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 4, 680–686 (2003).
MacLeod, M. K., Kappler, J. W. & Marrack, P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 130, 10–15 (2010).
Sabin, E. A., Araujo, M. I., Carvalho, E. M. & Pearce, E. J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 173, 269–272 (1996).
Malhotra, I. et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 162, 6843–6848 (1999).
Ndhlovu, P., Chimbari, M., Ndmba, J. & Chandiwana, S. K. 1992 National Schistosomiasis Survey: Blair Research Institute Report for Zimbabwe (Blair Research Institute, 1996).
Mutapi, F., Ndhlovu, P. D., Hagan, P. & Woolhouse, M. E. A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol 19, 255–263 (1997).
Fulford, A. C. J., Butterworth, A. E., Sturrock, R. F. & Ouma, J. H. On the use of age intensity data to detect immunity to parasitic infections, with special reference to schistosoma mansoni in Kenya. Parasitology 105, 219–227 (1992).
Anderson, R. M. & May, R. M. Macroparasites. Infectious diseases of humans: dynamics and control (Oxford Science, 1992).
Junge, S. et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol 37, 3270–3280 (2007).
Tanaskovic, S., Fernandez, S., Price, P., Lee, S. & French, M. A. CD31 (PECAM-1) is a marker of recent thymic emigrants among CD4+ T-cells, but not CD8+ T-cells or gammadelta T-cells, in HIV patients responding to ART. Immunol Cell Biol 88, 321–327 (2010).
Capron, M. & Capron, A. Immunoglobulin E and effector cells in schistomiasis. Science 264, 1876–1877 (1994).
Demeure, C. E. et al. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis 168, 1000–1008 (1993).
Hagan, P., Blumenthal, U. J., Dunne, D., Simpson, A. J. G. & Wilkins, A. H. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349, 234–245 (1991).
Maizels, R. M. & Yazdanbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3, 733–744 (2003).
Day, C. L. & Walker, B. D. Progress in defining CD4 helper cell responses in chronic viral infections. J Exp Med 198, 1773–1777 (2003).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188, 2205–2213 (1998).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205, 1797–1805 (2008).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Stephens, R. & Langhorne, J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog 6, e1001208 (2010).
Mutapi, F. et al. Age-related and infection intensity-related shifts in antibody recognition of defined protein antigens in a schistosome-exposed population. J Infect Dis 198, 167–175 (2008).
Black, C. L. et al. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis 202, 399–405 (2010).
Zaph, C. et al. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol 177, 511–518 (2006).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation and maintenance. Annu Rev Immunol 22, 745–763 (2004).
Fiuza, J. A. et al. Profile of central and effector memory T cells in the progression of chronic human chagas disease. PLoS Negl Trop Dis 3, e512 (2009).
Ben-Smith, A. et al. Differences between naive and memory T cell phenotype in Malawian and UK adolescents: a role for Cytomegalovirus? BMC Infect Dis 8, 139 (2008).
World Health Organisation. (ed. WHO) http://www.who.int/neglected_diseases/2010report/en/, Accessed January 2012, Geneva; 2011.
Wagatsuma, Y. et al. Resolution and resurgence of schistosoma haematobium-induced pathology after community-based chemotherapy in ghana, as detected by ultrasound. J Infect Dis 179, 1515–1522 (1999).
Chambers, C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 19, 565–594 (2001).
Taylor, M. D. et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol 179, 4626–4634 (2007).
Harari, A., Vallelian, F. & Pantaleo, G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol 34, 3525–3533 (2004).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8, 379–385 (2002).
Obst, R., van Santen, H. M., Mathis, D. & Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 201, 1555–1565 (2005).
Everts, B. et al. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis 4, e667 (2010).
Grogan, J. L., Kremsner, P. G., Deelder, A. M. & Yazdanbakhsh, M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J Infect Dis 177, 1433–1437 (1998).
Su, Z., Segura, M. & Stevenson, M. M. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect Immun 74, 2138–2144 (2006).
MacLeod, M. K. et al. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci U S A 105, 14521–14526 (2008).
Bangs, S. C. et al. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol 182, 1962–1971 (2009).
Roberts, M. et al. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infection and Immunity 61, 4984–4993 (1993).
World Health Organization in WHO technical Report Series (World Health Organisation, Geneva, 2002).
Mutapi, F. et al. Praziquantel treatment of people exposed to Schistosoma haematobium enhances serological recognition of defined parasite antigens. Journal of Infectious Diseases 192, 1108–1118 (2005).
Katz, N., Chaves, A. & Pellegrino, J. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev Inst MedTrop de Sao Paulo 14, 397–400 (1972).
Mott, K. E. A reusable polyamide filter for diagnosis of S. haematobium infection by urine filtration. Bull Soc Pathol Exot 76, 101–104 (1983).
Reilly, L. J., Magkrioti, C., Cavanagh, D. R., Mduluza, T. & Mutapi, F. Effect of treating Schistosoma haematobium infection on Plasmodium falciparum-specific antibody responses. BMC Infect Dis 8, 158 (2008).
Tchuente, L. A., Shaw, D. J., Polla, L., Cioli, D. & Vercruysse, J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J Trop Med Hyg 71, 778–782 (2004).
Imai, N. et al. Exposure, infection, systemic cytokine levels and antibody responses in young children concurrently exposed to schistosomiasis and malaria. Parasitology 138, 1519–1533 (2011).
Mutapi, F., Hagan, P., Ndhlovu, P. & Woolhouse, M. E. J. Comparison of humoral responses to Schistosoma haematobium in areas with high and low levels of infection. Parasite Immunology 19, 255–263 (1997).
Gil, M. E. & Coetzer, T. L. Real-time quantitative PCR of telomere length. Mol Biotechnol 27, 169–172 (2004).
Mutapi, F. & Roddam, A. p-values for pathogens: statistical inference from infectious-disease data. Lancet Infectious Disease 2, 219–230 (2002).
Sokal, R. R. & Rohlf, J. Biometry: the principles and practice of statistics in biological research (Freeman and Company, 1995).
Acknowledgements
First of all we would like to thank the residents, teachers and children in Magaya and Chipinda for their kind support of this study. We also very grateful the co-operation of the Ministry of Health and Child Welfare in Zimbabwe, the Provincial Medical Director of Mashonaland East, the Environmental Health Workers, nursing staff at Chitate and Chitowa Clinics and Murehwa Hospital. We also thank members of the National Institutes of Health in Zimbabwe and the Biochemistry Department at University of Zimbabwe for technical support. For help in performing experiments we like to thank Dana M.F. Photiou, Shu H. Choi. This work was supported by the World Health Organisation (Grant no RPC264), the Wellcome Trust (Grant no WT082028MA) and by Thrasher Research Funds.
Author information
Authors and Affiliations
Contributions
NN, NM, TM, FM designed the study. NN, OL, FT and FM conceived the experiments and NN performed the experiments. CDB, LJA, NN and FM were involved in the field work. NN and FM wrote the manuscript. OL and FT helped with manuscript redaction. All authors have reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Nausch, N., Bourke, C., Appleby, L. et al. Proportions of CD4+ memory T cells are altered in individuals chronically infected with Schistosoma haematobium. Sci Rep 2, 472 (2012). https://doi.org/10.1038/srep00472
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00472
This article is cited by
-
Role and Interrelationship Between Myeloid-Derived Suppressor Cells and CD4+ T Cells in Different Types of Infections: A Review
Proceedings of the Zoological Society (2024)
-
CD4+ memory T cells retain surface expression of CD31 independently of thymic function in patients with lymphoproliferative disorders following autologous hematopoietic stem-cell transplantation
International Journal of Hematology (2017)
-
Comprehensive Profiling of Peripheral Immune Cells and Subsets in Patients with Intermittent Allergic Rhinitis Compared to Healthy Controls and After Treatment with Glucocorticoids
Inflammation (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.