Abstract
Most cell death stimuli trigger the mitochondrial release of cytochrome c and other cofactors that induce caspase activation and ensuing apoptosis. Apoptosis is also associated with massive mitochondrial fragmentation and cristae remodeling. Dynamin-related protein 1 (Drp1), a protein of the mitochondrial fission machinery, has been reported to participate in apoptotic mitochondrial fragmentation. Several theories explaining the mechanisms of cytochome c release have been proposed. One suggests that it relies on the activation of Drp1-mediated mitochondrial fission. Here, we report that downregulation of Drp1 inhibits fragmentation of the mitochondrial network and partially prevents the release of cytochrome c but fails to prevent the release of other mitochondrial factors such as second mitochondria-derived activator of caspase/direct IAP-binding protein with low pI, Omi/HtrA2, adenylate kinase 2 and deafness dystonia peptide/TIMM8a. An explanation for the prevention of cytochrome c release is provided by our observation that inhibiting Drp1-mediated mitochondrial fission prevents the mitochondrial release of soluble OPA1 that was proposed to regulate cristae remodeling and complete cytochrome c release during apoptosis. Finally, we observed that downregulation of Drp1 delays but does not inhibit apoptosis, suggesting that mitochondrial fragmentation is not a prerequisite for apoptosis.
Similar content being viewed by others
Main
Programmed cell death (PCD) and its main phenotype, apoptosis, is a cellular suicide program essential for development and adult tissue homeostasis in all metazoan animals. Changes (inhibition or exacerbation) in PCD are involved in several pathogenic mechanisms including neurodegenerative diseases, cancers and AIDS. The stereotypical death throes of a cell undergoing apoptosis include DNA fragmentation, nuclear condensation, cell shrinkage, blebbing and phosphatidylserine (PS) externalization. These features are orchestrated by the activity of a family of cysteine proteases called caspases.1
A mitochondria-dependent step, involving mitochondrial outer membrane permeabilization (MOMP), is associated with most proapoptotic stimuli. This process is controlled by both pro- and anti-apoptotic members of the Bcl-2 family and leads to the cytosolic release of mitochondrial apoptogenic factors such as cytochrome c, second mitochondria-derived activator of caspase (Smac)/direct IAP-binding protein with low pI (DIABLO) and Omi/HtrA2.2 The mechanisms by which the proapoptotic Bcl-2 family members induce the release of mitochondrial proteins remain controversial.3 One model emphasizes rupture of the mitochondrial outer membrane as a consequence of mitochondrial swelling after the opening of the permeability transition pore. According to another model, proapoptotic Bcl-2 proteins like Bax/Bak induce a selective process of MOMP through the formation of channels or pores, allowing the release of proteins localized within the inner-membrane space such as cytochrome c. Finally, it has also been proposed that mitochondrial fragmentation is an additional, alternative or complementary mechanism in MOMP,4, 5 because mitochondria undergo rapid and massive fragmentation early in the apoptotic process.4, 6, 7
Mitochondria are dynamic organelles that continuously move, fuse and divide. Mammalian cells maintain mitochondrial shape by balancing the opposing processes of mitochondrial fusion and fission. The protein machinery that regulates mitochondrial fission and fusion has been well characterized and reviewed in detail.8 In mammalian cells, at least two proteins, dynamin-related protein 1 (Drp1) and hFis1, are required for mitochondrial fission.9, 10 Drp1 is a large GTPase that translocates to puncta on mitochondria, where it couples GTP hydrolysis with mitochondrial membrane constriction and fission.10, 11 How Drp1 is recruited to mitochondria to trigger fission is still unknown. However, one possibility is hFis1, a protein with a transmembrane domain that is anchored to the mitochondrial outer membrane via its C-terminal region.9 On the fusion side, mitofusins 1 and 2 (Mfn1 and 2) and OPA1 seem to be essential for the fusion of mitochondria.12, 13 These proteins are all large GTPases localized to mitochondria. Mfn1 and 2 reside within the outer membrane. However, OPA1 is an intermembrane space (IMS) protein with pools either soluble or closely associated with the inner membrane,14 and at least eight splicing isoforms have been reported.15 OPA1 is also thought to form oligomers involved in the regulation of mitochondrial cristae morphology and the complete release of cytochrome c during apoptosis.13, 14, 16
A transition from a mitochondrial network into vesicular punctiform mitochondria occurs invariably following apoptotic signals.4 Mitochondrial fragmentation is not inhibited by a broad caspase inhibitor but, interestingly, is inhibited by ectopic expression of a dominant-negative mutant of Drp1. More surprisingly, expression of the dominant-negative mutant of Drp1 not only inhibited mitochondrial fragmentation but also inhibited the release of cytochrome c and ensuing events such as the loss of mitochondrial membrane potential (ΔΨm) and nuclear DNA fragmentation.4 On the basis of these observations, it was therefore proposed that mitochondrial fragmentation (involving at least Drp1-mediated mitochondrial fission) is required for the release of cytochrome c and subsequent execution of apoptosis.4, 5 Nevertheless, some reports suggest that mitochondrial fragmentation follows the release of cytochrome c,14, 17, 18 and is largely unknown whether mitochondrial fragmentation is also required for the release of other mitochondrial factors. Moreover, whether mitochondrial fragmentation is indispensable for apoptosis execution is currently debated.19, 20, 21 Here, we report that inhibition of the mitochondrial fission by downregulating the expression of Drp1 through the use of RNA interference (RNAi) selectively prevents the release of cytochrome c during apoptosis. However, we observed that inhibition of Drp1-mediated fission only delays apoptosis, but it is not sufficient to prevent it.
Results
Inhibition of Drp1-mediated mitochondrial fission partially inhibits cytochrome c release but not that of other mitochondrial factors
Following Bax/Bak-mediated MOMP during apoptosis, several mitochondrial IMS proteins are released into the cytosol.2, 3 Among these are apoptogenic factors such as cytochrome c, Smac/DIABLO and Omi/HtrA2 that promote caspase activation and ensuing apoptosis.2 Bax/Bak-mediated MOMP also triggers the release of deafness dystonia peptide (DDP)/TIMM8a, a protein normally involved in the transport of transmembrane carrier proteins across the aqueous IMS to the inner mitochondrial membrane (IMM),22 into the cytoplasm where it binds to and promotes the mitochondrial localization of Drp1 to trigger fission.23 The release of adenylate kinase 2 (AK2) has also been described following Bax/Bak-mediated MOMP.24 As it has been reported that inhibition of Drp1-mediated fission prevents cytochrome c release during apoptosis,4, 6, 25, 26, 27 we examined whether the release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a were also prevented. For this purpose, downregulation of Drp1 by shRNAs was performed as described previously.26 Downregulation of Drp1 by RNAi was very efficient (Figure 1a), whereas actin levels remained unchanged (Figure 1a). As reported previously,26 the mitochondrial network in Drp1 RNAi-transfected cells was distinctively longer and more fused than in control RNAi-transfected cells (Figure 1b and c). Several reports indicate that mitochondria undergo rapid and massive fragmentation early in the apoptotic process, resulting in smaller, rounder and more numerous mitochondria.4, 6, 7 In agreement with previous studies,4, 7, 26, 28 we observed that disintegration of the mitochondrial network during apoptosis was prevented in cells in which Drp1-mediated fission was repressed (Figure 2b, lower panel).
Downregulation of Drp1 by RNAi induces mitochondrial fusion. HeLa cells were transfected with pREP4 constructs containing shRNA of the target sequence of Drp1, or else a control sequence, and the transfectants were selected by growth in media containing hygromycin B. (a) Total cell lysates from control RNAi and Drp1-RNAi transfected cells were prepared, and the expression level of Drp1 was analyzed by Western blotting. Actin level was also analyzed for a loading control. (b) Cells were transfected as in (a), and the percentage of cells with fused mitochondria was determined after mitochondrial staining with MitoTracker Red CMXRos. Data represent the means±S.D. of three independent experiments, with 300 cells per condition. Representative images are shown in (c), with the boxed areas enlarged in the lower panels
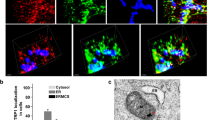
Knock down of Drp1 partially prevents cytochrome c release but not that of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a. (a) Control RNAi or Drp1 RNAi-transfected HeLa cells were treated for 18 and 24 h with 1 μM staurosporine (STS) or 10 μM actinomycin D (ActD). Next, cytosolic and heavy membrane fractions (containing mainly mitochondria) were analyzed by immunoblotting for the presence of DDP/TIMM8a (DDP), cytochrome c (Cyt c), Smac/DIABLO (Smac), AK2 and Omi/HtrA2 (Omi). As loading controls, actin was used for the cytosolic fraction and CoxIV for the heavy membrane fraction. (b) Control RNAi or Drp1 RNAi-transfected cells were either left untreated or else treated with 10 μM actinomycin D for 8 h in the presence of 50 μM zVAD-fmk to prevent apoptosis, then fixed, stained with anti-cytochrome c (green) and anti-Smac/DIABLO (red) antibodies, and analyzed by confocal microscopy. (c) Control RNAi or Drp1 RNAi-transfected HeLa cells were treated for 18 and 24 h with 1 μM STS or 10 μM actinomycin D (ActD). Next, total cell extract were analyzed by immunoblotting for caspase-3 processing and PARP cleavage
The mitochondrial release of cytochrome c, Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a was examined by Western blotting analysis of cytosolic and heavy membrane fractions (mainly mitochondria) from control RNAi- or Drp1 RNAi-transfected cells induced to undergo apoptosis by staurosporine or actinomycin D (Figure 2a); both drugs require Bax and/or Bak to trigger apoptosis.29 Mitochondrial release of cytochrome c, Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a occurred normally in control RNAi-tranfected cells undergoing apoptosis (Figure 2a). During apoptosis, we observed that mitochondria-associated Drp1 is cleaved by caspases (unpublished data). Therefore, less Drp1 is found in the heavy membrane fraction of control RNAi-transfected cells induced to undergo apoptosis than in untreated cells (Figure 2a). In Drp1 RNAi-transfected cells induced to undergo apoptosis, we observed that cytochrome c release was partially prevented but, interestingly, the mitochondrial efflux of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a was not affected because it occurred as in control RNAi-transfected cells (Figure 2a). Similarly, when our paper was in revision, another study has reported that downregulating Drp1 partially inhibits the release of cytochrome c from the mitochondria but fails to block the efflux of Smac/DIABLO.28
Curiously, although the mitochondrial release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a was not altered in Drp1 RNAi-transfected cells in comparison to control RNAi-transfected cells, detection of these mitochondrial factors was weaker in the cytosolic fraction of Drp1 RNAi-transfected cells (Figure 2a, top panel), suggesting that those proteins are degraded following their release. Why Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a might be degraded once they are released in the cytosol of Drp1 RNAi-transfected cells, but not in control RNAi-transfected cells, remains to be elucidated.
Immunocytochemistry experiments on untreated or actinomycin D-treated cells have also confirmed that inhibition of Drp1-mediated fission prevents cytochrome c release during apoptosis, as described previously,4, 6, 25, 26, 27 whereas Smac/DIABLO release is unaffected as it is found diffusely in the cytosol (Figure 2b, lower panel). In contrast, in actinomycin D-treated control RNAi-transfected cells, both cytochrome c and Smac/DIABLO are found diffusely in the cytosol (Figure 2b, top panel). Similar results were obtained substituting staurosporine for actinomycin D (data not shown).
As downregulation of Drp1 partially prevents cytochrome c release during apoptosis, we investigated whether it may also affect caspase activation, as cytochrome c is essential for caspase activation following Bax/Bak-mediated MOMP.2, 3 In total, cell extracts from control RNAi or Drp1 RNAi-transfected cells induced to undergo apoptosis, we analyzed caspase-3 activation, the main effector caspase in apoptosis1 and poly(ADP-ribose) polymerase (PARP) cleavage, a well-known caspase substrate. In agreement with the finding that cytochrome c release is partially prevented in staurosporine or actinomycin D-treated cells (Figure 2a), we observed that caspase-3 activation (assessed by the processing of its pro-form) and PARP cleavage were partially inhibited (Figure 2c).
Inhibiting Drp1-mediated mitochondrial fission induces respiratory defects
To find out why inhibition of Drp1-mediated fission prevents cytochrome c release, we studied mitochondrial respiration in intact cells, because inhibition of Drp1-mediated fission modifies the mitochondrial network morphology (Figure 1a)4, 7, 26 that may alter mitochondrial respiration. As cytochrome c serves as an electron shuttle in the respiratory chain, defects in respiration may explain the lack of cytochrome c release. Furthermore, knock down of proteins of the mitochondrial fusion machinery such as Mfns or OPA1 induces respiratory defects.30
Hence, oxygen consumption via complex I or complex II of the respiratory chain was studied in control RNAi or Drp1 RNAi-transfected cells using specific substrates of each complex. Although oxygen consumption in complex I of control RNAi or Drp1 RNAi-transfected cells is similar (Figure 3a and b), we observed that oxygen consumption in complex II of Drp1 RNAi-transfected cells was significantly decreased in comparison to control cells (Figure 3a and b). It, therefore, appears that knock down of Drp1 modifies the cell respiration by affecting complex II of respiratory chain.
Drp1-depleted cells have defects in complex II of the respiratory chain. (a) Representative respiration profiles through complex I (top) or II (bottom) of the respiratory chain of control RNAi or Drp1-RNAi-transfected cells. First arrows denote when substrates were added in the chamber and second when inhibitor was added. Rates of oxygen depletion (nmol/ml/min) are indicated in each profile. (b) Bar graph showing complex II respiratory defect in Drp1-RNAi transfected cells. Errors bars represent S.D.
Cytochrome c release is selectively inhibited from mitochondria of Drp1-depleted cells
Respiration is altered in Drp1 RNAi-transfected cells (Figure 3), and thus it may modify cellular energy homeostasis. To rule out the possibility that the prevention of cytochrome c release in Drp1-depleted cells might be due to changes in cellular homeostasis, the release of mitochondrial protein was assessed in vitro using isolated mitochondria from control or Drp1 RNAi-depleted cells. In vitro incubation of isolated mitochondria with recombinant tBid triggers a rapid and complete release of cytochrome c, Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a in mitochondria from control cells (Figure 4, left panel). tBid requires either Bax or Bak to induce MOMP.29 Interestingly, we observed that cytochrome c release is inhibited when we used isolated mitochondria from cells in which expression of Drp1 has been knocked down (Figure 4, right panel). This lack of cytochrome c release might be due to an inhibition of Bax/Bak-mediated MOMP. Nevertheless, in agreement with our results obtained in cells, knock down of Drp1 had no detectable effects on the release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a (Figure 4, right panel), confirming that Bax/Bak-mediated MOMP had occurred. Hence, it appears that the prevention of cytochrome c release from Drp1-depleted mitochondria is because of intrinsic changes in mitochondria.
Cytochrome c release is selectively prevented from mitochondrial isolated from Drp1-depleted cells. Mitochondria isolated from control RNAi or Drp1 RNAi-transfected HeLa cells were incubated with (+) or without (−) 10 nM tBid at 30°C for different time points. Mitochondrial and supernatant fractions were resolved by SDS-PAGE and immunoblotted with antibodies against the indicated proteins. Equal loading of the mitochondrial pellet lanes was monitored using anti-VDAC antibody
Inhibiting Drp1-mediated mitochondrial fission delays but does not prevent apoptosis and cell death
The observation that knock down of Drp1 reduces cytochrome c release and caspase activation (Figure 2) suggests that Drp1 depletion may protect against apoptosis and cell death. To check this possibility, staurosporine and actinomycin-induced apoptosis were assessed at different time in control RNAi and Drp1 RNAi-transfected cells. Apoptosis was assessed using flow cytometry by measuring DNA degradation, mitochondrial membrane potential (ΔΨm) loss and PS exposure – three apoptotic hallmarks. As shown in Figure 5, inhibiting Drp1-mediated mitochondrial fission slightly delayed but did not prevent apoptosis induced either by staurosporine or actinomycin D, in agreement with a study28 published when our paper was in revision. Moreover, cell viability was studied and similarly, knock down of Drp1 delayed but did not prevent cell death (Figure 5). As a positive control, cells were pretreated with zVAD-fmk, a synthetic broad-spectrum caspase inhibitor, before staurosporine or actinomycin D treatment. In both cases, caspase inhibition inhibited significantly both apoptosis and cell death (data not shown).
Downregulation of Drp1 delays but does not inhibit apoptosis and cell death. Control RNAi or Drp1 RNAi-transfected HeLa cells were treated for 6, 9, 18, 24, 30 and 36 h with 1 μM staurosporine or 10 μM actinomycin D. DNA degradation, ΔΨm loss and PS exposure, three apoptotic hallmarks, were then assessed by flow cytometry. Cell viability (plasma membrane integrity) was also studied by flow cytometry. The data are expressed as means±S.D. of three independent experiments, ns: not significant
Inhibiting Drp1-mediated mitochondrial fission modifies the pattern of OPA1 isoforms and prevents its release during apoptosis
It has been proposed that a mitochondrial cristae rearrangement is required to allow the complete release of cytochrome c during apoptosis because most cytochrome c (>80%) is normally sequestered within mitochondrial cristae folds.31 In agreement with this hypothesis, it was recently shown that the dynamin-like GTPase OPA1 not only participates in mitochondrial fusion machinery but also regulates inner membrane cristae reorganization to facilitate the rapid and complete cytochrome c release during apoptosis.14, 16 In addition, the mitochondrial rhomboid protease PARL cleaves OPA1 to produce short IMS soluble isoforms involved in the regulation of cristae morphology and complete cytochrome c release.32 The short IMS soluble isoforms of OPA1 form hetero-oligomers with longer isoforms of OPA1. These hetero-oligomers regulate the tightness of cristae junctions and are disrupted soon after MOMP, leading to the opening of cristae junctions and full cytochrome c release.16
The overall morphology of the mitochondrial population depends on the relative activities of the fission and fusion machinery. Hence, inhibition of Drp1-mediated fission may affect effectors of fusion machinery such as OPA1. In agreement with this hypothesis, we observed that inhibition of Drp1-mediated fission modifies the pattern of OPA1 isoforms; knock down of Drp1 induces the loss of the two higher OPA1 isoforms and an increase in the shorter isoforms (Figure 6a) that have been demonstrated to regulate the tightness of cristae junction.16 Further studies are required to assess whether inhibition of Drp1-mediated fission promotes the processing of OPA1 by PARL as recently proposed.32 However, other proteases may be involved because the m-AAA protease paraplegin was also reported to regulate proteolytic cleavage of OPA1.33
Drp1 RNAi modifies the pattern of OPA1 isoforms and prevents OPA1 release during apoptosis. (a) Total cell lysates from untreated or control RNAi and Drp1-RNAi transfected cells were prepared, and Drp1 and OPA1 were analyzed by immunoblotting. Actin was used as a loading control. The high and short soluble isoforms of OPA1 are indicated. (b) Control RNAi or Drp1 RNAi-transfected cells were either left untreated or else treated with 10 μM actinomycin D for 8 h in the presence of 50 μM zVAD-fmk to prevent apoptosis, then fixed, stained with anti-OPA1 (green) and anti-Smac/DIABLO (red) antibodies, and analyzed by confocal microscopy
We have reported a co-release of OPA1 with cytochrome c following Bax/Bak-mediated MOMP (Supplementary Figure S1), and we have proposed that this mitochondrial release of short, soluble OPA1 isoforms participates in cristae remodeling to allow the release of previously sequestered cytochrome c.14 Given that knock down of Drp1 modifies the pattern of OPA1 isoforms (Figure 6a), we consequently considered whether inhibiting Drp1-mediated fission might also inhibit the release of soluble OPA1 isoforms, preventing cristae remodeling and the complete release of cytochrome c. In control RNAi-transfected cells, OPA1 and Smac/DIABLO were both diffusely present in the cytosol when cells were treated with actinomycin D (Figure 6b, top panel). In contrast, in actinomycin D-treated Drp1-depleted cells, Smac/DIABLO release still occurred, whereas OPA1 remained within the mitochondria (Figure 6b). Similar results were obtained substituting staurosporine for actinomycin D (data not shown). Because in Drp1-depleted cells, an increase of the short, soluble OPA1 isoforms is observed (Figure 6a), the hetero-oligomers that regulate the tightness of cristae junction are likely not disrupted after MOMP preventing, therefore, the release of soluble OPA1 isoforms but more importantly, the opening of the cristae junction and ensuing full cytochrome c release. Together, our findings suggest that inhibiting Drp1-mediated mitochondrial fission prevents cytochrome c release by increasing the short IMS soluble OPA1 isoforms that regulate cristae remodeling and ensuing complete release of cytochrome c during apoptosis.14, 16
Discussion
In this study, we show that inhibition of Drp1-mediated mitochondrial fission partially prevents cytochrome c release but has no effect on the release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a in cells induced to undergo apoptosis. Similarly, in vitro studies using mitochondria from Drp1-depleted cells exposed to tBid revealed that cytochrome c release is inhibited, whereas the release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a is unaffected. Thus, the differential release of cytochrome c and other mitochondrial IMS proteins seems to result from an intrinsic change in mitochondria from Drp1 RNAi-transfected cells.
It has been proposed that mitochondrial cristae rearrangement is required to allow the complete release of cytochrome c during apoptosis31 because most cytochrome c (>80%) is normally sequestered within mitochondrial cristae folds. Although it is known that most cytochrome c is normally sequestered within mitochondrial cristae folds, the intracompartment localization of the IMS proteins Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a is not known. Are they also localized in both the IMS proper as well as within the sequestered cristae folds-like cytochrome c, or just within the unsequestered IMS? This is an area under current investigation in our laboratory, but it has been reported that Smac/DIABLO, Omi/HtrA2 are both soluble within the IMS.34 The observation that the release of Smac/DIABLO, Omi/HtrA2, AK2 and DDP/TIMM8a is not prevented when Drp1 is knocked down suggests that those factors, unlike cytochrome c, are not ‘trapped’ within the cristae folds, and therefore their release does not require cristae rearrangement.31 OPA1, a protein of the fusion machinery, has been recently shown to also regulate mitochondrial cristae morphology and the complete cytochrome c release during apoptosis.14, 16 Following Bax/Bak-MOMP, short soluble isoforms of OPA1 that specifically regulate the tightness of cristae junction are co-released with IMS cytochrome c, leading to cristae opening and release of remaining cytochrome c stored within the cristae folds.14, 16 In this study, we observed that knock down of Drp1 not only prevents cytochrome c release but also the release of OPA1 soluble isoforms, probably as a consequence of modifications in the pattern of OPA1 isoforms. Therefore, inhibiting Drp1-mediated fission seems to prevent cytochrome c release by preventing the OPA1-regulated cristae remodeling required for the complete cytochrome c release. Nevertheless, inhibition of cytochrome c release in Drp1-depleted cells appears to be ‘collateral damage’ resulting from the long and fused mitochondrial network that affects effectors of the fusion machinery such as OPA1 also involved in maintaining cristae morphology. Finally, we do not rule out the possibility that inhibiting Drp1-mediated mitochondrial fission may also affect other unidentified proteins involved in cristae morphology and/or cytochrome c release.
It has also been proposed that, before its release, cytochrome c must be solubilized in the IMS, because cytochrome c is present as loosely and tightly bound pools attached to the inner IMM through its association with cardiolipin35 or other electrostatic interactions.34 During apoptosis, cardiolipin peroxidation has been reported to be required for the cytochrome c detachment from IMM and its full release.35, 36 Depletion of Drp1 in cells may decrease the rate of cardiolipin peroxidation in apoptosis and thus its mitochondrial release following MOMP. Moreover, as we observed that Drp1-depleted cells show a defect in the complex II of the respiratory chain, we also do not exclude the possibility that prevention of cytochrome c release may be because of this change.
In this study, we also report that inhibiting Drp1-mediated mitochondrial fission delays, but does not prevent Bax/Bak-mediated apoptosis and cell death, although it partially prevents cytochrome c release and caspase activation. Our results are in agreement with two other time-course studies demonstrating that knock down of Drp1 delays but does not inhibit apoptosis.27, 28 Furthermore, it was recently shown that Caenorhabditis elegans Bcl-2 related protein CED-9 expression in mammalian cells prevents apoptosis-associated mitochondrial fission/fragmentation, whereas it has no effects on cytochrome c release and apoptosis.37 In contrast with our results, it was shown in two earlier studies, that inhibition of Drp1-mediated mitochondrial fission prevents apoptosis,4, 26 but their conclusions were based on assessment of apoptosis at single time point. Still, we do not rule out the possibility that, in their models, complete cytochrome c release is required to induce caspase activation and ensuing apoptosis. Indeed, it has been reported that the levels of cytosolic cytochrome c required to trigger caspase activation differ in a cell type-dependent manner.38
In conclusion, although results from previous studies suggest that Drp1-mediated mitochondrial fission is required for cytochrome c release4, 6, 25, 26, 27 and apoptosis execution,4, 26 we observed that knock down of Drp1 selectively and partially prevents cytochrome c release, and that it delays but does not protect against Bax/Bak-mediated apoptosis. Thus, according to our results, Drp1-mediated fission appears dispensable and does not seem to be a prerequisite for apoptosis.
Materials and Methods
Cell culture and reagents
HeLa cells were cultured under standard conditions in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), supplemented with 10% fetal calf serum, 2 mM L-glutamine, 50 IU penicillin and 50 μg/ml streptomycin. Transfection of HeLa cells was performed using FuGENE 6 (Roche Applied Science). zVAD-fmk and MitoTracker Red CMXRos were purchased from Calbiochem and Molecular Probes, respectively. Actinomycin D and staurosporine were purchased from Sigma-Aldrich.
RNA interference
RNAi was performed using the sh-activated gene silencing system. Plasmids expressing shRNAs were constructed by synthesizing two cDNA oligonucleotides bearing the target sequence, HindIII linker, and U6 terminator, annealing them, and ligating them into BseRI and BamHI sites of pSHAG-1 (Cold Spring Harbor Laboratory). For long-term suppression of gene expression by shRNA, the region encoding the U6 promoter and shRNA in pSHAG-1 was subcloned into the NotI and BamHI sites of pREP4 (Invitrogen, Carlsbad, CA). The target sequence for Drp1 was as follows: 5′-TTCAATCCGTGATGAGTATGCTTTTCTTC-3′. The sequence for the control shRNA was 5′-TCGTACTCATAATCAGCTCTGCATACATC-3′, which does not target any genes. One day after the transfection with the pREP4 construct, HeLa cells were grown in DMEM containing 300 μg/ml hygromycin B for 2 days, followed by 3–4 days in DMEM containing 50 μg/ml hygromycin B for the selection of transfectants.
Subcellular fractionation and immunoblotting
HeLa cells were harvested in isotonic mitochondrial buffer (MB: 210 mM mannitol, 70 mM sucrose, 1 mM EDTA and 10 mM HEPES; (pH 7.5), supplemented with the protease inhibitor mixture Complete (Roche Molecular Biochemicals) and homogenized for 30–40 strokes with a Dounce homogenizer. Samples were transferred to Eppendorf centrifuge tubes and centrifuged (500 × g, 5 min, 4°C) to remove nuclei and unbroken cells. The resulting supernatant was then centrifuged (10 000 × g, 30 min, 4°C) to obtain the heavy membrane fraction enriched for mitochondria; the resulting supernatant was collected as the cytosolic fraction. Cytosolic and heavy membranes fractions (30 and 10 μg of protein, respectively) were resolved by SDS-PAGE (10–20% Tricine gels; Novex) and transferred to nitrocellulose membranes (Amersham Biosciences). After blocking nonspecific sites for 1 h at room temperature with 5% nonfat milk and 0.2% Tween 20 in phosphate-buffered saline (pH 7.4), the membrane was incubated with rabbit polyclonal anti-DDP/TIMM8a23 (1 : 500 dilution), mouse monoclonal anti-cytochrome c (BD Biosciences Pharmingen, clone 7H8.2C12) (1 : 2000), rabbit polyclonal anti-Smac/DIABLO (ProScience) (1 : 1000), rabbit polyclonal anti-adenylate kinase 2 (Santa Cruz) (1 : 1000), rabbit polyclonal anti-Omi/HtrA239 (1 : 2000), mouse monoclonal anti-Drp1/DLP1 (BD Biosciences Pharmingen, clone 8) (1 : 1000) or mouse monoclonal anti-OPA1 (BD Biosciences Pharmingen, clone 18) (1 : 1000) antibodies. To confirm equal protein loading and transfer, membranes were subsequently reprobed with anti-actin (Sigma-Aldrich, clone AC-40) (1 : 5000) and anti-Cox IV monoclonal antibodies (Molecular Probes, clone 10G8) (1 : 3000) as indicated. After treatment with horseradish peroxidase-linked goat anti-mouse or anti-rabbit secondary antibodies (1 : 4000; Amersham Biosciences), immunoreactive proteins were detected using enhanced chemiluminescence (ECL; Amersham Biosciences). For caspase-3 and PARP detection, cells were lysed in Laemmli buffer, and cell lysates were resolved by SDS-PAGE (NuPAGE 4–12% Bis–Tris gel; Novex) and transferred to nitrocellulose membranes. After blocking nonspecific sites for 1 h at room temperature with 5% nonfat milk and 0.2% Tween 20 in phosphate-buffered saline (pH 7.4), the membranes were incubated with rabbit polyclonal anti-caspase 3 (Stressgen) (1 : 2000) and mouse monoclonal anti-PARP (BD Biosciences Pharmingen, clone C2–10) (1 : 3000) antibodies.
Immunofluorescence microscopy
Cells grown in LabTek chambers were fixed for 10 min in 4% paraformaldehyde followed by permeabilization with 0.15% Triton X-100 in phosphate-buffered saline for 15 min. The cells were then incubated for 1 h in blocking buffer (2% bovine serum albumin (BSA) in phosphate-buffered saline) followed by incubation overnight with either a mouse monoclonal anti-cytochrome c (BD Biosciences Pharmingen, clone 6H2.B4) (1 : 800) antibody or mouse monoclonal anti-OPA1 (BD Biosciences Pharmingen, clone 18) (1 : 2000) and rabbit polyclonal anti-Smac/DIABLO (ProScience) (1 : 600). Cells were washed three times for 10 min each in blocking buffer, then incubated for 2 h with Alexa Fluor anti-mouse and anti-rabbit secondary antibodies (Molecular Probes). Images were acquired using a Zeiss LSM 510 confocal microscope through a × 63 oil fluorescence objective (Carl Zeiss Inc.).
Isolation of mitochondria and in vitro assays for the release of mitochondrial factors
Mitochondria were isolated intact from HeLa cells by sucrose density gradient centrifugation. Briefly, cells were harvested in phosphate-buffered saline containing 5 mM EDTA, centrifuged at 750 × g for 10 min, washed and resuspended in isotonic MB supplemented with protease inhibitors. Cells were broken by five passages through a 25-gauge needle fitted onto a 5 ml syringe, and the suspension was then centrifuged at 2000 × g at 4°C. This procedure was repeated until nearly all of the cells were broken. Supernatants from each step were pooled before centrifugation (13 000 × g, 10 min, 4°C). The resulting pellet containing mitochondria was resuspended in 1 ml of MB and layered on top of a discontinuous sucrose gradient consisting of 19 ml of 1.2 M sucrose, 1 mM EDTA and 0.1% BSA in 10 mM HEPES (pH 7.5) over 16 ml of 1.6 M sucrose, 1 mM EDTA and 0.1% BSA in 10 mM HEPES (pH 7.5). Samples were centrifuged (27 000 rpm, 2 h, 4°C) in a Beckman SW28 rotor. Mitochondria were recovered at the 1.6/1.2 M sucrose buffer interface, washed and resuspended in MB.
Isolated mitochondria (30 μg) were incubated in the presence or absence of 10 nM caspase-8-cleaved recombinant human Bid (tBid; R&D Systems) in 200 μl of KCl buffer (125 mM KCl, 4 mM MgCl2, 5 mM NaHPO4, 5 mM succinate, 0.5 mM EGTA, 15 mM HEPES–KOH (pH 7.4) and 5 μM rotenone) for various time point at 30°C, and the mitochondria were recovered by centrifugation (13 000 × g, 5 min, 4°C). Aliquots of mitochondrial pellets (5 μg protein) and the corresponding volume of the supernatant fractions were subjected to SDS-PAGE using 10–20% Tricine gels (Novex) and their respective contents of mitochondrial proteins were estimated by immunoblotting as described above. Consistent loading of the mitochondrial pellet was verified using anti-VDAC mouse monoclonal antibody (Calbiochem; clone 31HL) (1 : 6000).
Oxygen electrode polarography
Oxygen consumption was measured in intact cells using Clark's electrode (Hansatech). In brief, cells (1 mg/ml) were incubated in 400 μl respiration buffer (100 mM KMES, 5 mM KH2PO4, 1 mM EGTA, 1 mM EDTA, 1 mg/ml BSA, 1 mM ADP, pH 7.4) under mixing. Next cells were permeabilized with 10 μl digitonin 0.2% and oxygen consumption via complex I was assessed by adding 10 mM pyruvate, 5 mM malate and 10 mM malonate. To assess oxygen consumption via complex II, 20 mM succinate and 25 μM rotenone (inhibitor of complex I) were added. In both conditions, after few minutes of oxygen consumption, respiration was blocked with 30 mM KCN.
Flow cytometric analysis
Mitochondrial transmembrane potential (ΔΨm) was measured by incubating cells with 3,3′-dihexyloxacarbicyanine iodide – DiOC6(3) – (Molecular Probes) at a concentration of 50 nM. Plasma membrane integrity (cell viability) was assessed with 1 μg/ml propidium iodide (Molecular Probes). PS exposure on the outer plasma membrane was measured by staining cells with 1 μg/ml of annexin V-FITC (Becton Dickinson). To evaluate DNA degradation, cells were incubated with the nuclear dye acridine orange (0.1 μg/ml; Molecular Probes). Cells were analyzed using a FACScan (Becton Dickinson).
Statistical analyses
Data were compared using Student's t-test. Differences were considered to be significant if P<0.05.
Abbreviations
- AK2:
-
adenylate kinase 2
- DDP:
-
deafness dystonia peptide
- DIABLO:
-
direct IAP-binding protein with low pI
- Drp1:
-
dynamin-related protein 1
- IMM:
-
inner-mitochondrial membrane
- IMS:
-
intermembrane space
- Mfn:
-
mitofusin
- MOMP:
-
mitochondrial outer membrane permeabilization
- PARP:
-
poly(ADP-ribose) polymerase
- PCD:
-
programmed cell death
- Smac:
-
second mitochondria-derived activator of caspase
References
Riedl SJ, Shi Y . Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004; 5: 897–907.
Wang X . The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15: 2922–2933.
Green DR, Kroemer G . The pathophysiology of mitochondrial cell death. Science 2004; 305: 626–629.
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 2001; 1: 515–525.
Youle RJ, Karbowski M . Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 2005; 6: 657–663.
Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC . Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 2003; 160: 1115–1127.
Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 2002; 159: 931–938.
Okamoto K, Shaw JM . Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 2005; 39: 503–536.
James DI, Parone PA, Mattenberger Y, Martinou JC . hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 2003; 278: 36373–36379.
Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM . A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 1998; 143: 351–358.
Smirnova E, Griparic L, Shurland DL, van der Bliek AM . Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001; 12: 2245–2256.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC . Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 2003; 160: 189–200.
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 2003; 278: 7743–7746.
Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C . Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem 2005; 280: 35742–35750.
Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L et al. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet 2001; 109: 584–591.
Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006; 126: 177–189.
Esseiva AC, Chanez AL, Bochud-Allemann N, Martinou JC, Hemphill A, Schneider A . Temporal dissection of Bax-induced events leading to fission of the single mitochondrion in Trypanosoma brucei. EMBO Rep 2004; 5: 268–273.
Gao W, Pu Y, Luo KQ, Chang DC . Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J Cell Sci 2001; 114: 2855–2862.
Arnoult D . Mitochondrial fragmentation in apoptosis. Trends Cell Biol 2007; 17: 6–12.
Martinou JC, Youle RJ . Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ 2006; 13: 1291–1295.
Parone PA, Martinou JC . Mitochondrial fission and apoptosis: an ongoing trial. Biochim Biophys Acta 2006; 1763: 522–530.
Bauer MF, Hofmann S, Neupert W, Brunner M . Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol 2000; 10: 25–31.
Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J et al. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol 2005; 15: 2112–2118.
Single B, Leist M, Nicotera P . Simultaneous release of adenylate kinase and cytochrome c in cell death. Cell Death Differ 1998; 5: 1001–1003.
Germain M, Mathai JP, McBride HM, Shore GC . Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 2005; 24: 1546–1556.
Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ . Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 2004; 15: 5001–5011.
Sugioka R, Shimizu S, Tsujimoto Y . Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 2004; 279: 52726–52734.
Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol 2006; 26: 7397–7408.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Chen H, Chomyn A, Chan DC . Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 2005; 280: 26185–26192.
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell 2002; 2: 55–67.
Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 2006; 126: 163–175.
Ishihara N, Fujita Y, Oka T, Mihara K . Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 2006; 25: 2966–2977.
Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM . Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J Biol Chem 2005; 280: 2266–2274.
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S . Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA 2002; 99: 1259–1263.
Petrosillo G, Ruggiero FM, Pistolese M, Paradies G . Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett 2001; 509: 435–438.
Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ . Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell 2006; 21: 761–773.
Brustugun OT, Fladmark KE, Doskeland SO, Orrenius S, Zhivotovsky B . Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ 1998; 5: 660–668.
Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ . Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 2003; 22: 4385–4399.
Acknowledgements
We are very grateful to Dr. Anne Lombès and for her valuable assistance in oxygen consumption studies and to Dr. Craig blackstone for critical reviews of our manuscript, respectively. We thank Dr. Richard Youle for the gift or control RNAi and Drp1 RNAi constructs and for discussions. Special thanks to Dr. Philippe Parone and Dr. Jean Claude Martinou for stimulating scientific discussions and for sharing unpublished data. We apologize to the authors of relevant articles that are not cited because of space limitations. This work was funded by grants from the Agence Nationale de Recherche sur le Sida (ANRS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by DR Green
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Estaquier, J., Arnoult, D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ 14, 1086–1094 (2007). https://doi.org/10.1038/sj.cdd.4402107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402107
Keywords
This article is cited by
-
Calcineurin inhibition protects against dopamine toxicity and attenuates behavioral decline in a Parkinson’s disease model
Cell & Bioscience (2023)
-
The centrosomal protein 131 participates in the regulation of mitochondrial apoptosis
Communications Biology (2023)
-
Mitochondrial dynamics in health and disease: mechanisms and potential targets
Signal Transduction and Targeted Therapy (2023)
-
A narrative review of the effects of dexamethasone on traumatic brain injury in clinical and animal studies: focusing on inflammation
Inflammopharmacology (2023)
-
Liver Injury Induced by Exposure to Polystyrene Microplastics Alone or in Combination with Cadmium in Mice Is Mediated by Oxidative Stress and Apoptosis
Biological Trace Element Research (2023)