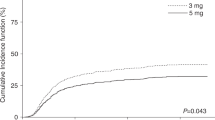
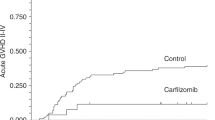
Abstract
Cyclosporin remains the most widely used immunosuppressive agent in patients undergoing allogeneic stem cell transplantation (SCT). The increased awareness of the impact of the intensity of post-transplant immunosuppression on determining outcome after allogeneic SCT has resulted in a re-examination of whether cyclosporin is currently being optimally used in this population of patients. Recent studies in solid organ transplantation have questioned whether the use of trough levels provides the most accurate reflection of the immunosuppressive actions of cyclosporin and alternative strategies to monitor cyclosporin dosage after liver and kidney transplantation are increasingly being used. As a result there is now interest in examining whether there is scope for translating these advances into the arena of haematopoietic transplantation. In this paper, we will review the rationale underlying the current schedules for dosing and monitoring cyclosporin after allogeneic SCT and identify specific areas in which the use of cyclosporin requires re-evaluation. These include evaluation of whether patient outcome would be improved by using peak cyclosporin levels to determine dosing schedules, analysis of optimal cyclosporin dosing schedules in patients undergoing reduced intensity allografts and investigation of surrogate markers of cyclosporin's immunosuppressive activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Powles RL, Clink HM, Spence D, Morgenstern G, Watson JG, Selby PJ et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet 1980; 16: 327–329.
Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukaemia: long-term follow up of a controlled trial. Blood 1989; 73: 1729–1734.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303–2314.
Nash RA, Antin JA, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation. Blood 2000; 96: 2062–2068.
Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 181–185.
Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant 2004; 34: 621–625.
Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB et al. Sirolimus, tacrolimus and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor malignancies. Blood 2003; 102: 1601–1605.
Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplantation 2004; 10: 328–336.
Ruutu T, Niederwieser D, Gratwohl A, Apperley JF . A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 1997; 19: 759–764.
Duncan N . A survey of practice relating to the dosing, administration and monitoring of i.v. ciclosporin in UK haematology centers. British Oncology Pharmacy Association Annual Symposium; Birmingham, 2004.
Bacigalupo A, Van Lint MT, Occhini D, Gualandi T, Lamparelli T, Sogno E et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukaemia. Blood 1991; 77: 1423–1428.
Bacigalupo A, Lamparelli T, Gualandi F, Bregante S, Raiola A, di Grazia C et al. Increased risk of leukaemia relapse with high dose cyclosporine after allogeneic marrow transplantation for acute leukaemia: 10-year follow-up of a randomized study. Blood 2001; 98: 3174–3175.
Byrne JL, Stainer C, Hyde H, Miflin G, Haynes AP, Bessell EM et al. Low incidence of acute graft-versus-host disease and recurrent leukaemia in patients undergoing allogeneic haemopoietic stem cell transplantation from sibling donors with methotrexate and dose-monitored cyclosporin A prophylaxis. Bone Marrow Transplant 1998; 22: 541–545.
Mengarelli A, Iori AP, Romano A, Cerretti R, Cerilli L, De Propris MS et al. One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allongeneic peripheral blood stem cell transplantation. Haematologica 2003; 88: 315–323.
Yu C, Storb R, Mathey B, Deeg HJ, Schuening FG, Graham TC et al. DLA-identical bone marrow grafts after low-dose total body irradiation: effects of high-dose corticosteroids and cyclosporine on engraftment. Blood 1995; 86: 4376–4381.
Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol 2005; 23: 9387–9393.
Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978; ii: 1323.
Kahan BD . Cyclosporine. N Engl J Med 1989; 321: 1725–1738.
Halloran PF, Helms LM, Kung L, Noujaim J . The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation 1999; 68: 1356–1361.
Ptachcinski RJ, Venkataramanan R, Burckart GJ . Clinical pharmacokinetics of ciclosporin. Clin Pharmacokinetics 1986; 11: 107–132.
Kahan BD . Therapeutic drug monitoring of cyclosporine: 20 years of progress. Transplant Proc 2004; 36 (S1): S378–S391.
Wood AJ, Maurer G, Neiderberger W, Beveridge T . Cyclosporine: pharmacokinetics, metabolism and drug interactions. Transplant Proc 1983; 15: 2409–2412.
Atkinson K, Biggs JC, Britton K, Short R, Mrongovius R, Concannon A et al. Oral administration of cyclosporin A for recipients of allogeneic marrow transplantation: implications of clinical gut dysfunction. Br J Haematol 1984; 56: 223–231.
Yee GC, Lennon TP, Gmur DG, Carlin J, Schaffer RL, Kennedy MS et al. Clinical pharmacology of cyclosporine in patients undergoing bone marrow transplantation. Transplant Proc 1986; 18: 153–159.
Freeman DJ, Laupacis A, Keown PA, Stiller CR, Carruthers SG . Evaluation of cyclosporin–phenytoin interaction with observations on cyclosporin metabolites. Br J Clin Pharmacol 1984; 18: 887–893.
Kramer MR, Marshall SE, Denning DW, Keogh AM, Tucker RM, Galgiani JN et al. Cyclosporine and itraconazole interaction in heart and lung transplant recipients. Ann Intern Med 1990; 113: 327–329.
Canafax DM, Graves NM, Hilligoss DM, Carleton BC, Gardner MJ, Matas AJ . Interaction between cyclosporine and fluconazole in renal allograft recipients. Transplantation 1991; 51: 1014–1018.
Ptachcinski RJ, Carpenter BJ, Burckhart GJ, Venkataramanan R, Rosenthal JT . Effect of erythromycin on cyclosporine levels. N Engl J Med 1985; 313: 1416–1417.
Spicer ST, Liddle C, Chapman JR, Barclay P, Nankivell BJ, Thomas P et al. The mechanism of cyclosporine toxicity induced by clarithromycin. Br J Clin Pharmacol 1997; 43: 194–196.
Citterio F . Evolution of the therapeutic drug monitoring of cyclosporine. Transplant Proc 2004; 36: 420S–425S.
Schmidt H, Ehninger G, Dopfer R, Blaurock M, Naumann R, Einsele H et al. Correlation between low CSA plasma concentration and severity of acute GvHD in bone marrow transplantation. Blut 1988; 57: 139–142.
Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ . Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med 1988; 319: 65–70.
Przepiorka D, Shapiro S, Schwinghammer TL, Bloom EJ, Rosenfeld CS, Shadduck RK et al. Cyclosporine and methylprednisolone after allogeneic marrow transplantation: association between low cyclosporine concentration and risk of acute graft-versus-host disease. Bone Marrow Transplant 1991; 7: 461–465.
Ghalie R, Fitzsimmons WE, Weinstein A, Manson S, Kaizer H . Cyclosporine monitoring improves graft-versus-host-disease prophylaxis after bone marrow transplantation. Ann Pharmacother 1994; 28: 379–383.
Barrett AJ, Kendra JR, Lucas CF, Joss DV, Joshi R, Pendharkar P et al. Cyclosporin A as prophylaxis against graft-versus-host disease in 36 patients. BMJ (Clin Res Ed) 1982; 285: 162–166.
Gratwohl A, Speck B, Wenk M, Forster I, Muller M, Osterwalder B et al. Cyclosporine in human bone marrow transplantation. Serum concentration, graft-versus-host disease and nephrotoxicity. Transplantation 1983; 36: 40–44.
Bacigalupo A, Di Georgio F, Frassoni F, VanLint MT, Raffo MR, Gogioso L et al. Cyclosporin A serum and blood levels in marrow graft recipients: correlation with administered dose, serum creatinine and graft-versus host disease. Acta Haematol 1984; 72: 155–162.
Kennedy Ms, Yee GC, McGuire TR, Leonard TM, Crowley JJ, Deeg HJ . Correlation of serum cyclosporine concentrations with renal dysfunction in marrow transplant recipients. Transplantation 1985; 40: 249–253.
Yee GC, Kennedy MS, Gmur DJ, Self SG, Deeg HJ . Monitoring cyclosporin concentrations in marrow transplant recipients: comparison of two assay methods. Bone Marrow Transplant 1987; 1: 289–295.
Kahan BD, Wideman CA, Reid M, Gibbons S, Jarowenko M, Flechner S et al. The value of serial serum trough cyclosporine levels in human renal transplantation. Transplant Proc 1984; 16: 1195–1199.
Holt DW, Marsden JT, Johnston A, Bewick M, Taube DH . Blood cyclosporin concentrations and renal allograft dysfunction. BMJ (Clin Res Ed) 1986; 293: 1057–1059.
Nankivell BJ, Hibbins M, Chapman JR . Diagnostic utility of whole blood cyclosporine measurements in renal transplantation using triple therapy. Transplantation 1994; 58: 989–996.
Kahan BD, Grevel J . Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation 1988; 46: 631–644.
White D, Rose M, Wright L, Wallwork J, English TA, Hakim M et al. Failure of whole blood cyclosporine levels to provide a reliable measure of immunosuppression in clinical heart and heart/lung transplantation. Transplant Proc 1988; 20: 422–425.
Henry ML, Bowers VD, Fanning WJ, Sommer BG, Ferguson RM . Cyclosporine levels are not helpful. Transplant Proc 1988; 20: 419–421.
Mahalati K, Belitsky P, Sketris I, West K, Panek R . Neoral monitoring by simplified sparse sampling area under the concentration–time curve. Transplantation 1999; 68: 55–62.
Grant D, Kneteman N, Tchervenkov J, Roy A, Murphy G, Tan A et al. Peak cyclosporine levels (Cmax) correlate with freedom from liver graft rejection. Transplantation 1999; 67: 1133–1137.
Cantarovich M, Barkun JS, Tchervenkov J, Besner JG, Aspeslet L, Metrakos P . Comparison of neoral dose monitoring with cyclosporine trough levels versus 2-hr post dose levels in stable liver transplant patients. Transplantation 1998; 66: 1621–1627.
International Neoral Renal Transplantation Study Group. Randomized, international study of cyclosporine microemulsion absorption profiling in renal transplantation with basiliximab immunoprophylaxis. Am J Transplant 2002; 2: 157–166.
Morris RG, Russ GR, Cervelli MJ, Juneja R, McDonald SP, Mathew TH . Comparison of trough, 2-hour and limited AUC blood sampling for monitoring cyclosporin (Neoral) at day 7 post-renal transplantation and incidence of rejection in the first month. Ther Drug Monit 2002; 24: 479–486.
Grevel J, Walsh MS, Kahan BD . Cyclosporine monitoring in renal transplantation: area under the curve monitoring is superior to trough-level monitoring. Ther Drug Monit 1989; 11: 246–248.
Lindholm A, Kahan BD . Influence of cyclosporine pharmacokinetics, trough concentration and AUC monitoring on outcome after kidney transplantation. Clin Pharmacol Ther 1993; 54: 205–218.
Meyer MM, Munar M, Udeaja J, Bennett W . Efficacy of area under the curve cyclosporine monitoring in renal transplantation. J Am Soc Nephrol 1993; 4: 1306–1315.
Johnston A . Sparse-sampling – a practical method for measurement of AUCs. Focus on Med 1998; 13: 7–10.
Johnston A, Sketris I, Marsden JT, Galustian CG, Fashola T, Taube D et al. A limited sampling strategy for the measurement of cyclosporine AUC. Transplant Proc 1990; 22: 1345–1346.
Cantarovich M, Besner JG, Barkun JS, Elstein E, Loertscher R . Two-hour cyclosporine level determination is the appropriate tool to monitor Neoral therapy. Clin Transplantation 1998; 12: 243–249.
Levy G . Two-hour cyclosporin concentration (C2) as a monitoring tool for Neoral. Focus on Med 1998; 13: 19–22.
Cantarovich M, Elstein E, De Varennes D, Barkun JS . Clinical benefit of neoral dose monitoring with cyclosporine 2-hr post dose levels compared with trough levels in stable heart transplant patients. Transplantation 1999; 68: 1839–1842.
Canadian Neoral Renal Transplantation Study Group. Absorption profiling of cyclosporine microemulsion (neoral) during the first 2 weeks after renal transplantation. Transplantation 2001; 72: 1024–1032.
Levy G, Burra P, Cavallari A, Duvoux C, Lake J, Mayer AD et al. Improved clinical outcomes for liver transplant recipients using cyclosporine monitoring based on 2-hr post-dose levels (C2). Transplantation 2002; 73: 953–959.
Levy G, Thervet E, Lake J, Uchida K . Patient management by neoral C2 monitoring: an international consensus statement. Transplantation 2002; 73: S12–S18.
Holt DW, Armstrong VW, Griesmacher A, Morris RG, Napoli KL, Shaw LM . International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology Working Group on Immunosuppressive Drug Monitoring. Therap Drug Monitor 2002; 24: 59–67.
Pai SY, Fruman DA, Leong T, Neuberg D, Rosano TG, McGarigle C et al. Inhibition of calcineurin phosphatase activity in adult bone marrow transplant patients treated with cyclosporin A. Blood 1994; 84: 3974–3979.
Sanquer S, Schwarzinger M, Maury S, Yakouben K, Rafi H, Pautas C et al. Calcineurin activity as a therapeutic index of immunosuppression: a functional, pharmacodynamic approach for GVHD prophylaxis. Transplantation 2004; 77: 854–858.
Delaney MP, Smythe E, Higgins RM, Morris AG . Constitutive and acquired resistance to calcineurin inhibitors in renal transplantation: role of P-glycoprotein-170. Transpl Int 2000; 13: 276–284.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duncan, N., Craddock, C. Optimizing the use of cyclosporin in allogeneic stem cell transplantation. Bone Marrow Transplant 38, 169–174 (2006). https://doi.org/10.1038/sj.bmt.1705404
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705404
Keywords
This article is cited by
-
Analysis of the variable factors affecting changes in the blood concentration of cyclosporine before and after transfusion of red blood cell concentrate
Journal of Pharmaceutical Health Care and Sciences (2022)
-
Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and Calliphora vicina
Scientific Reports (2021)
-
Low cyclosporine concentrations in children and time to acute graft versus host disease
BMC Pediatrics (2020)
-
Converting cyclosporine A from intravenous to oral administration in hematopoietic stem cell transplant recipients and the role of azole antifungals
European Journal of Clinical Pharmacology (2018)
-
Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches
Cellular & Molecular Immunology (2017)