Summary:
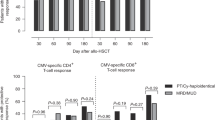
We studied the incidence and recurrence of Cytomegalovirus (CMV) infection and reactivation in 38 recipients of Alemtuzumab reduced intensity conditioning-stem cell transplantation, and used CMV-HLA tetramer studies to discover if these events correlated with recovery of circulating CMV-specific CD8+ T cells (cytotoxic T lymphocyte (CTLs)). The cumulative incidence of CMV infection was 60% at 1 year (95% CI, 45–78%) with a median reactivation time of 24 days (range 5–95 days). All patients with CMV reactivation received Ganciclovir or Foscarnet, and only one developed CMV disease. More strikingly, only 8/21 patients had relapse of CMV antigenemia. Tetramer analysis in 13 patients showed that 11 reconstituted CMV CTLs (7/11 by day 30 and 10/11 by day 90). The development of CMV infection was accompanied by a >5-fold rise of CMV CTLs. Recurrence of CMV infection occurred only in the patients who failed to generate a CTL response to the virus. Hence, recipients of SCT using Alemtuzumab-RIC are initially profoundly immunosuppressed and have a high incidence of early CMV reactivation. However, in the majority of patients, infection is transient, and antiviral T cell reconstitution is rapid. Monitoring with CMV-specific CTLs may help identify the subset of patients at risk from recurrent infection or disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Carella AM, Champlin R, Slavin S et al. Mini-allografts: ongoing trials in humans. Bone Marrow Transplant 2000; 25: 345–350.
Feinstein L, Sandmaier B, Maloney D et al. Nonmyeloablative hematopoietic stem cell transplantation. Replacing high-dose cytotoxic therapy by the graft-versus-tumor effect. Ann N Y Acad Sci 2001; 938: 328–337.
Friedman TM, Varadi G, Hopely DD et al. Nonmyeloablative conditioning allows for more rapid T cell repertoire reconstitution following allogeneic matched unrelated bone marrow transplantation compared to myeloablative approaches. Biol Blood Marrow Transplant 2001; 7: 656–664.
Morecki S, Gelfand Y, Nagler A et al. Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs myeloablative regimen. Bone Marrow Transplant 2001; 28: 243–249.
Bornhauser M, Thiede C, Platzbecker U et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res 2001; 7: 2254–2262.
Maris MB, Niederwieser D, Sandmaier BM et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003; 102: 2021–2030.
Nagler A, Aker M, Or R et al. Low-intensity conditioning is sufficient to ensure engraftment in matched unrelated bone marrow transplantation. Exp Hematol 2001; 29: 362–370.
Wong R, Giralt SA, Martin T et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood 2003; 102: 3052–3059.
Boeckh M . Current antiviral strategies for controlling cytomegalovirus in hematopoietic stem cell transplant recipients: prevention and therapy. Transpl Infect Dis 1999; 1: 165–178.
Krance RA, Kuehnle I, Rill DR et al. Hematopoietic and immunomodulatory effects of lytic CD45 monoclonal antibodies in patients with hematologic malignancy. Biol Blood Marrow Transplant 2003; 9: 273–281.
Nitsche A, Steuer N, Schmidt C et al. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin Chem 1999; 45: 1932–1937.
Hale G, Zhang MJ, Bunjes D et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood 1998; 92: 4581–4590.
Hale G, Jacobs P, Wood L et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant 2000; 26: 69–76.
Klangsinsirikul P, Carter GI, Byrne JL et al. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood 2002; 99: 2586–2591.
Bindon CI, Hale G, Clark M, Waldmann H . Therapeutic potential of monoclonal antibodies to the leukocyte-common antigen. Synergy and interference in complement-mediated lysis. Transplantation 1985; 40: 538–544.
Wulf GG, Luo KL, Goodell MA, Brenner MK . Anti-CD45-mediated cytoreduction to facilitate allogeneic stem cell transplantation. Blood 2003; 101: 2434–2439.
Morris EC, Rebello P, Thomson KJ et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood 2003; 102: 404–406.
DelleKarth G, Laczika K, Scholten C et al. Clearance of PCR-detectable lymphoma cells from the peripheral blood, but not bone marrow after therapy with Campath-1H. Am J Hematol 1995; 50: 146–147.
Weinblatt ME, Maddison PJ, Bulpitt KJ et al. Campath-1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis: an intravenous dose-escalation study. Arthritis Rheum 1995; 38: 1589–1594.
Matteson EL, Yocum DE, St Clair EW et al. Treatment of active refractory rheumatoid arthritis with humanized monoclonal antibody Campath-1H administered by daily subcutaneous injection. Arthritis Rheum 1995; 38: 1187–1193.
Brett S, Baxter G, Cooper H et al. Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, Campath-1H. Immunology 1996; 88: 13–19.
Keating MJ, Flinn I, Jain V et al. Therapeutic role of alemtuzumab (CAMPATH-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Martino R, Caballero MD, Canals C et al. Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2001; 28: 341–347.
Junghanss C, Boeckh M, Carter RA et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood 2002; 99: 1978–1985.
Chakrabarti S, Mackinnon S, Chopra R et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath – 1H in delaying immune reconstitution. Blood 2002; 99: 4357–4363.
Cwynarski K, Ainsworth J, Cobbold M et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood 2001; 97: 1232–1240.
Davison GM, Novitzky N, Kline A et al. Immune reconstitution after allogeneic bone marrow transplantation depleted of T cells. Transplantation 2000; 69: 1341–1347.
Riddell SR, Watanabe KS, Goodrich JM et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992; 257: 238.
Acknowledgements
The Amy Strelzer Manasevit Scholar Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lamba, R., Carrum, G., Myers, G. et al. Cytomegalovirus (CMV) infections and CMV-specific cellular immune reconstitution following reduced intensity conditioning allogeneic stem cell transplantation with Alemtuzumab. Bone Marrow Transplant 36, 797–802 (2005). https://doi.org/10.1038/sj.bmt.1705121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705121
Keywords
This article is cited by
-
Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment
Nature Communications (2024)
-
Early detection of cytomegalovirus-specific cytotoxic T lymphocytes against cytomegalovirus antigenemia in human leukocyte antigen haploidentical hematopoietic stem cell transplantation
Annals of Hematology (2015)
-
Allogeneic stem cell transplantation using alemtuzumab-containing regimens in severe aplastic anemia
International Journal of Hematology (2013)
-
Fludarabine-based reduced intensity conditioning transplants have a higher incidence of cytomegalovirus reactivation compared with myeloablative transplants
Bone Marrow Transplantation (2010)
-
Alemtuzumab markedly reduces chronic GVHD without affecting overall survival in reduced-intensity conditioning sibling allo-SCT for adults with AML
Bone Marrow Transplantation (2009)