Summary:
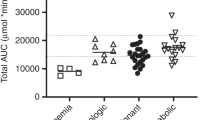
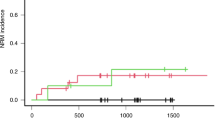
A strong relationship has been demonstrated between high systemic exposure to busulfan and the occurrence of hepatic veno-occlusive disease (HVOD) after a busulfan–cyclophosphamide regimen (BU CY). We report a prospective study aimed at exploring the pharmacodynamics of high-dose busulfan combined with either melphalan (BU MEL) or thiotepa (BU TTP) followed by autologous stem cell transplantation in children and adolescents with a malignant solid tumor. Busulfan was given orally at a total dose of 600 mg m−2. In all, 45 patients with a median age of 6.3 years were included in the study: 25 received BU MEL and 20 received BU TTP. The incidence of HVOD was 44% (CI 95% [23–65%]) in the BU MEL group and 25% (CI95% [9–49%]) in the BU TTP group. In the BU TTP group, patients who developed HVOD had a significantly higher AUC 0-6 h after the 13th dose (6201±607 h ng ml−1) than those who did not (5024±978 h ng ml−1) (P<0.05). In the BU MEL group, there was no difference in terms of systemic exposure to busulfan between patients who developed HVOD and those who did not. In conclusion, the guidelines established for monitoring BU CY cannot be extrapolated when busulfan is combined with another drug.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dunn CD . The chemical and biological properties of busulphan (“Myleran”). Exp Hematol 1974; 2: 101–117.
Santos GW, Tutschka PJ, Brookmeyer R et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Lenarsky C, Kohn DB, Weinberg KI, Parkman R . Bone marrow transplantation for genetic diseases. Hematol Oncol Clin North Am 1990; 4: 589–602.
Lenarsky C, Parkman R . Bone marrow transplantation for the treatment of immune deficiency states. Bone Marrow Transplant 1990; 6: 361–369.
Santos GW . The development of busulfan/cyclophosphamide preparative regimens. Semin Oncol 1993; 20: 12–16.
Hartmann O, Valteau-Couanet D, Vassal G et al. Prognostic factors in metastatic neuroblastoma in patients over 1 year of age treated with high-dose chemotherapy and stem cell transplantation: a multivariate analysis in 218 patients treated in a single institution. Bone Marrow Transplant 1999; 23: 789–795.
Valteau-Couanet D, Benhamou E, Vassal G et al. Consolidation with a busulfan-containing regimen followed by stem cell transplantation in infants with poor prognosis stage 4 neuroblastoma. Bone Marrow Transplant 2000; 25: 937–942.
Kalifa C, Hartmann O, Demeocq F et al. High-dose busulfan and thiotepa with autologous bone marrow transplantation in childhood malignant brain tumors: a phase II study. Bone Marrow Transplant 1992; 9: 227–233.
Dupuis-Girod S, Hartmann O, Benhamou E et al. Will high dose chemotherapy followed by autologous bone marrow transplantation supplant cranio-spinal irradiation in young children treated for medulloblastoma? J Neurooncol 1996; 27: 87–98.
DeLeve LD, Shulman HM, McDonald GB . Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–42.
Bearman SI . Avoiding hepatic veno-occlusive disease: what do we know and where are we going? Bone Marrow Transplant 2001; 27: 1113–1120.
Meresse V, Hartmann O, Vassal G et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141.
Grochow LB, Jones RJ, Brundrett RB et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Hassan M, Ehrsson H, Ljungman P . Aspects concerning busulfan pharmacokinetics and bioavailability. Leuk Lymphoma 1996; 22: 395–407.
Grochow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20: 18–25.
Slattery JT, Sanders JE, Buckner CD et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics [published erratum appears in Bone Marrow Transplant 1996 Oct; 18(4): 829]. Bone Marrow Transplant 1995; 16: 31–42.
Bolinger AM, Zangwill AB, Slattery JT et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Sandstrom M, Karlsson MO, Ljungman P et al. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplant 2001; 28: 657–664.
Bolinger AM, Zangwill AB, Slattery JT et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Slattery JT, Risler LJ . Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit 1998; 20: 543–549.
Bleyzac N, Souillet G, Magron P et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant 2001; 28: 743–751.
Vassal G, Koscielny S, Challine D et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–253.
McDonald GB, Hinds MS, Fisher LD et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Vassal G, Re M, Gouyette A . Gas chromatographic-mass spectrometric assay for busulfan in biological fluids using a deuterated internal standard. J Chromatogr 1988; 428: 357–361.
Vassal G, Deroussent A, Challine D et al. Is 600 mg/m2 the appropriate dosage of busulfan in children undergoing bone marrow transplantation? Blood 1992; 79: 2475–2479.
Shumaker RC . PKCALC: a BASIC interactive computer program for statistical and pharmacokinetic analysis of data. Drug Metab Rev 1986; 17: 331–348.
Hassan M, Oberg G, Bekassy AN et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol 1991; 28: 130–134.
Grochow LB, Krivit W, Whitley CB, Blazar B . Busulfan disposition in children. Blood 1990; 75: 1723–1727.
Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J . Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol 1989; 24: 386–390.
Dix SP, Wingard JR, Mullins RE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Schuler U, Schroer S, Kuhnle A et al. Busulfan pharmacokinetics in bone marrow transplant patients: is drug monitoring warranted? Bone Marrow Transplant 1994; 14: 759–765.
Slattery JT, Kalhorn TF, McDonald GB et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol 1996; 14: 1484–1494.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
McCune JS, Gooley T, Gibbs JP et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Vassal G, Tranchand B, Valteau-Couanet D et al. Pharmacodynamics of tandem high-dose melphalan with peripheral blood stem cell transplantation in children with neuroblastoma and medulloblastoma. Bone Marrow Transplant 2001; 27: 471–477.
Vesole DH, Crowley JJ, Catchatourian R et al. High-dose melphalan with autotransplantation for refractory multiple myeloma: results of a Southwest Oncology Group phase II trial. J Clin Oncol 1999; 17: 2173–2179.
Czerwinski M, Gibbs JP, Slattery JT . Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos 1996; 24: 1015–1019.
Gibbs JP, Czerwinski M, Slattery JT . Busulfan–glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res 1996; 56: 3678–3681.
Paumi CM, Ledford BG, Smitherman PK et al. Role of multidrug resistance protein 1 (MRP1) and glutathione S-transferase A1-1 in alkylating agent resistance. Kinetics of glutathione conjugate formation and efflux govern differential cellular sensitivity to chlorambucil versus melphalan toxicity. J Biol Chem 2001; 276: 7952–7956.
Vahrmeijer AL, van Dierendonck JH, Schutrups J et al. Potentiation of the cytostatic effect of melphalan on colorectal cancer hepatic metastases by infusion of buthionine sulfoximine (BSO) in the rat: enhanced tumor glutathione depletion by infusion of BSO in the hepatic artery. Cancer Chemother Pharmacol 1999; 44: 111–116.
Anderson CP, Tsai J, Chan W et al. Buthionine sulphoximine alone and in combination with melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur J Cancer 1997; 33: 2016–2019.
Shulman HM, Luk K, Deeg HJ et al. Induction of hepatic veno-occlusive disease in dogs. Am J Pathol 1987; 126: 114–125.
DeLeve LD, Wang X . Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology 2000; 60: 143–154.
Hassan M, Ljungman P, Ringden O et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915–924.
Coles BF, Morel F, Rauch C et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics 2001; 11: 663–669.
Nakajima T, Elovaara E, Anttila S et al. Expression and polymorphism of glutathione S-transferase in human lungs: risk factors in smoking-related lung cancer. Carcinogenesis 1995; 16: 707–711.
Balasubramanian P, Chandy M, Krishnamoorthy R, Srivastava A . Evaluation of existing limited sampling models for busulfan kinetics in children with beta thalassaemia major undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 821–825.
Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS . Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol 1996; 37: 401–408.
Andersson BS, Madden T, Tran HT et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Acknowledgements
This study was supported by a grant from the ‘comité de recherche clinique’, Institut Gustave Roussy. We thank all the nursing staff of the bone marrow transplantation unit ‘La Mer’, Department of Paediatrics Oncology, Institut Gustave Roussy. We are grateful to Lorna Saint Ange for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouligand, J., Boland, I., Valteau-Couanet, D. et al. In children and adolescents, the pharmacodynamics of high-dose busulfan is dependent on the second alkylating agent used in the combined regimen (melphalan or thiotepa). Bone Marrow Transplant 32, 979–986 (2003). https://doi.org/10.1038/sj.bmt.1704275
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704275
Keywords
This article is cited by
-
Busulfan systemic exposure and its relationship with efficacy and safety in hematopoietic stem cell transplantation in children: a meta-analysis
BMC Pediatrics (2020)
-
Veno-occlusive disease after high-dose busulfan–melphalan in neuroblastoma
Bone Marrow Transplantation (2020)
-
The pharmacokinetics and pharmacodynamics of busulfan when combined with melphalan as conditioning in adult autologous stem cell transplant recipients
Annals of Hematology (2018)
-
Busulfan–melphalan in high-risk neuroblastoma: the 30-year experience of a single institution
Bone Marrow Transplantation (2016)
-
Busulfan–Melphalan followed by autologous stem cell transplantation in patients with high-risk neuroblastoma or Ewing sarcoma: an exposed–unexposed study evaluating the clinical impact of the order of drug administration
Bone Marrow Transplantation (2016)