Summary:
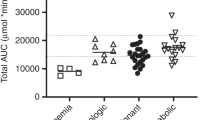
Busulfan (Bu) is an important component of some myeloablative regimens prior to stem cell transplantation (SCT). Over the last few years it has been shown that other drugs administered concomitantly can influence Bu pharmacokinetics. In the present study, we compared Bu concentrations (trough levels) in three groups of patients. Group A (n=5) received metronidazole as graft-versus-host disease prophylaxis during Bu treatment. Group B (n=9) received Bu only for 2 days followed by 2 days of Bu and metronidazole. Group C (n=10) was a control group that received Bu without metronidazole. The mean Bu levels for Group A receiving metronidazole during conditioning was significantly (P<0.001) higher (948±280 ng/ml), compared to those observed in the control group (507±75 ng/ml). In Group B, the administration of metronidazole resulted in a significant (P<0.001) increase in Bu levels (807±90 ng/ml) during the last 2 days, compared to 452±68 ng/ml during the first 2 days. In Group A, one patient died with multiorgan failure, three experienced veno-occlusive disease (VOD) and one developed hemorrhagic cystitis. Elevated liver transaminases (AST, ALT) and bilirubin were detected in all Group A patients. In Group B, six patients had elevated liver function tests but no VOD was observed. We conclude that metronidazole should not be administered simultaneously with Bu to avoid the high plasma levels of Bu, which may lead to severe toxicity and/or treatment related mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Socie G, Clift RA, Blaise D et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood 2001; 98: 3569–3574.
Bolinger AM, Zangwill AB, Slattery JT et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Hassan M, Ljungman P, Bolme P et al. Busulfan bioavailability. Blood 1994; 84: 2144–2150.
Vassal G, Fischer A, Challine D et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood 1993; 82: 1030–1034.
Vassal G, Deroussent A, Challine D et al. Is 600 mg/m2 the appropriate dosage of busulfan in children undergoing bone marrow transplantation? Blood 1992; 79: 2475–2479.
Hassan M, Oberg G, Bjorkholm M et al. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol 1993; 33: 181–186.
Buggia I, Zecca M, Alessandrino EP et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo). Anticancer Res 1996; 16: 2083–2088.
Hassan M, Svensson JO, Nilsson C et al. Ketobemidone may alter busulfan pharmacokinetics during high-dose therapy. Ther Drug Monit 2000; 22: 383–385.
Grochow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20: 18–25; quiz 26.
Vassal G, Koscielny S, Challine D et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–253.
Vergnon JM, Boucheron S, Riffat J et al. Interstitial pneumopathies caused by busulfan. Histologic, developmental and bronchoalveolar lavage analysis of 3 cases. Rev Med Intern 1988; 9: 377–383.
Ljungman P, Hassan M, Bekassy AN et al. Busulfan concentration in relation to permanent alopecia in recipients of bone marrow transplants. Bone Marrow Transplant 1995; 15: 869–871.
Chattergoon DS, Saunders EF, Klein J et al. An improved limited sampling method for individualised busulphan dosing in bone marrow transplantation in children. Bone Marrow Transplant 1997; 20: 347–354.
Hassan M, Fasth A, Gerritsen B et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant 1996; 18: 843–850.
Zander AR, Zabelina T, Kroger N et al. Use of a five-agent GVHD prevention regimen in recipients of unrelated donor marrow. Bone Marrow Transplant 1999; 23: 889–893.
Beelen DW, Elmaagacli A, Muller KD et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood 1999; 93: 3267–3275.
Samuelson J . Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother 1999; 43: 1533–1541.
Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow trans-plantation for leukemia. N Engl J Med 1986; 314: 729–735.
Ringden O, Remberger M, Persson U et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 1995; 15: 619–625.
Hassan M, Ehrsson H . Gas chromatographic determination of busulfan in plasma with electron-capture detection. J Chromatogr 1983; 277: 374–380.
Jones RJ, Lee KS, Beschorner WE et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Vassal G, Deroussent A, Hartmann O et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res 1990; 50: 6203–6207.
Hassan Z, Ljungman P, Ringden O et al. Pharmacokinetics of liposomal busulphan in man. Bone Marrow Transplant 2001; 27: 479–485.
Olavarria E, Hassan M, Eades A et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. Leukemia 2000; 14: 1954–1959.
Andersson BS, Madden T, Tran HT et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Schuler US, Renner UD, Kroschinsky F et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol 2001; 114: 944–950.
Loft S . Metronidazole and antipyrine as probes for the study of foreign compound metabolism. Pharmacol Toxicol 1990; 66: 1–31.
Levy RH . Cytochrome P450 isozymes and antiepileptic drug interactions. Epilepsia 1995; 36: S8–S13.
Loft S, Poulsen HE . Metabolism of metronidazole and antipyrine in isolated rat hepatocytes. Influence of sex and enzyme induction and inhibition. Biochem Pharmacol 1989; 38: 1125–1136.
Kazmier FJ . A significant interaction between metronidazole and warfarin. Mayo Clin Proc 1976; 51: 782–784.
van der Weide J, Steijns LS, van Weelden MJ et al. The effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics 2001; 11: 287–291.
Spina E, Pisani F, Perucca E . Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet 1996; 31: 198–214.
Hassan M, Ehrsson H . Metabolism of 14C-busulfan in isolated perfused rat liver. Eur J Drug Metab Pharmacokinet 1987; 12: 71–76.
Marchand DH, Remmel RP, Abdel-Monem MM . Biliary excretion of a glutathione conjugate of busulfan and 1,4-diiodobutane in the rat. Drug Metab Dispos 1988; 16: 85–92.
Gibbs JP, Yang JS, Slattery JT . Comparison of human liver and small intestinal glutathione S-transferase-catalyzed busulfan conjugation in vitro. Drug Metab Dispos 1998; 26: 52–55.
Gibbs JP, Murray G, Risler L et al. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res 1997; 57: 5509–5516.
Hassan M, Oberg G, Ehrsson H et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol 1989; 36: 525–530.
Hassan M, Ehrsson H . Urinary metabolites of busulfan in the rat. Drug Metab Dispos 1987; 15: 399–402.
Damani LA, Houdi AA . Cytochrome P-450 and FAD-monooxygenase mediated S- and N-oxygenations. Drug Metabol Drug Interact 1988; 6: 235–244.
Sherratt AJ, Banet DE, Linder MW et al. Potentiation of 3-methylcholanthrene induction of rat hepatic cytochrome P450IA1 by dexamethasone in vivo. J Pharmacol Exp Ther 1989; 249: 667–672.
Larsson P, Cybulski W, Tjalve H . Binding of 3H-metronidazole in olfactory, respiratory and alimentary epithelia in rats. Pharmacol Toxicol 1997; 81: 65–73.
Martelli A, Allavena A, Robbiano L et al. Comparison of the sensitivity of human and rat hepatocytes to the geno-toxic effects of metronidazole. Pharmacol Toxicol 1990; 66: 329–334.
Hassan M, Ljungman P, Ringden O et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915–924.
Shulman HM, Luk K, Deeg HJ et al. Induction of hepatic veno-occlusive disease in dogs. Am J Pathol 1987; 126: 114–125.
Acknowledgements
The authors express their gratitude to the Swedish Children Cancer Society (BCF) grant no. PROJ 01/059, King Gustav V Jubilee fund grant no. 00:510 and Stockholms Cancer Foundation grant no. 02:119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nilsson, C., Aschan, J., Hentschke, P. et al. The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 31, 429–435 (2003). https://doi.org/10.1038/sj.bmt.1703896
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703896
Keywords
This article is cited by
-
Vitamin D levels and busulphan kinetics in patients undergoing hematopoietic stem cell transplantation, a multicenter study
Bone Marrow Transplantation (2021)
-
Predicting the presence and mechanism of busulfan drug-drug interactions in hematopoietic stem cell transplantation using pharmacokinetic interaction network–based molecular structure similarity and network pharmacology
European Journal of Clinical Pharmacology (2021)
-
Busulfan-cyclophosphamide versus cyclophosphamide-busulfan as conditioning regimen before allogeneic hematopoietic cell transplantation: a prospective randomized trial
Annals of Hematology (2021)
-
Intra-individual Pharmacokinetic Variability of Intravenous Busulfan in Hematopoietic Stem Cell-Transplanted Children
Clinical Pharmacokinetics (2020)
-
Clostridium difficile Infection (CDI) in Solid Organ and Hematopoietic Stem Cell Transplant Recipients
Current Infectious Disease Reports (2014)