Abstract
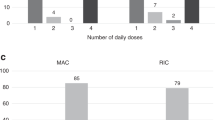
Autologous recovery is a major problem with busulfan as a marrow ablative agent in conditioning children for allogeneic BMT. Data suggest the average concentration of busulfan at steady state (Bu Css) is critical for successful engraftment. We prospectively evaluated busulfan pharmacokinetics in 31 children (age 0.6–18 years) with AML (n = 9), and non-malignant diseases (n = 22) receiving HLA-closely matched (sibling, parent, unrelated) donor grafts. Blood samples were obtained following dose 1 and 13 of a standard 16 dose, 4-day regimen. The busulfan dose varied from 14 to 20 mg/kg. Patients received cyclophosphamide 200–240 mg/kg; 22/31 received 80–90 mg/kg of ATG. Eight patients failed to engraft (26%). ATG did not appear to influence engraftment (P = 0.38). Bu Css levels <600 ng/ml correlated with autologous recovery/mixed chimerism (P = 0.018). there were no graft failures in patients with a bu css >600 ng/ml. a correlation between bu css levels and regimen-related toxicity (rrt) was not identified for grade 2 or higher toxicities, only 1/31 had a bu css >900 ng/ml. Our data support the use of pharmacokinetic monitoring of busulfan. Bone Marrow Transplantation (2000) 25, 925–930.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tutschka PJ, Santos GW . Bone marrow transplantation in the busulfan-treated rat: relationship between myelosuppression and immunosuppression for conditioning bone marrow recipients Transplantation 1977 24: 52–62
Santos GW, Tutschka PJ . Marrow transplantation in the busulfan treated rat: preclinical model of aplastic anemia J Natl Cancer Inst 1974 53: 1781–1785
Peters WP, Henner WB, Growchow LB et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors Cancer Res 1987 47: 6402–6406
Grochow LB, Krivit W, Whitley CB, Blazer BR . Busulfan disposition in children Blood 1990 75: 1723–1727
Hassan M, Oberg G, Bekassy AN et al. Pharmacokinetics of high dose busulphan in relation to age and chronopharma-cology Cancer Chemother Pharmacol 1991 28: 130–134
Vassal G, Gouyette A, Hartmann O et al. Pharmacokinetics of high dose busulfan in children Cancer Chemother Pharmacol 1989 24: 386–390
Gibbs JP, Murray G, Risler L et al. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan Cancer Res 1997 57: 5509–5516
Vassal G, Deroussent A, Challine D et al. Is 600 mg/m2 the appropriate dosage of busulfan in children undergoing bone marrow transplantation? Blood 1992 79: 2475–2479
Hassan M, Ljungman P, Bolme P et al. Busulfan bioavailability Blood 1994 84: 2144–2150
Slattery JT, Clift RA, Buckner CD et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation Blood 1997 89: 3055–3060
Slattery JT, Sanders JE, Buckner CD et al. Graft rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics Bone Marrow Transplant 1995 16: 31–42
Dix SP, Wingard JR, Mullins RE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT Bone Marrow Transplant 1996 17: 225–230
Growchow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens Semin Oncol 1993 20: 18–25
Santos GW, Tutschka PJ, Brookmeyer R et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide New Engl J Med 1983 309: 1347–1353
Slattery JT, Risler LJ . Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation Ther Drug Monit 1998 20: 543–549
Bearman SI, Applelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation J Clin Oncol 1988 6: 562–568
Agresti A . An Introduction to Categorical Data Analysis Wiley: New York 1996
Cleveland WS . Robust locally weighted regression and smoothing scatter plots J Am Stat Assoc 1979 74: 829–836
Regazzi MB, Locatelli F, Buggia I et al. Disposition of high-dose busulfan in pediatric patients undergoing bone marrow transplantation Clin Pharmacol Ther 1993 53: 45–52
Pawlowska AB, Blazar BR, Angelucci E et al. Relationship of plasma pharmacokinetics of high-dose oral busulfan to the outcome of allogeneic bone marrow transplantation in children with thalassemia Bone Marrow Transplant 1997 20: 915–920
Tutschka PJ, Copelan EA, Klein JP . Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen Blood 1987 70: 1382–1388
Acknowledgements
This work was supported in part by NIH grant M01-RR01271, Pediatric Clinical Research Center; NIH grant CA18029, Children’s Cancer Research Fund of the University of Minnesota.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bolinger, A., Zangwill, A., Slattery, J. et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 25, 925–930 (2000). https://doi.org/10.1038/sj.bmt.1702371
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702371
Keywords
This article is cited by
-
Busulfan systemic exposure and its relationship with efficacy and safety in hematopoietic stem cell transplantation in children: a meta-analysis
BMC Pediatrics (2020)
-
Population pharmacokinetic analysis of intravenous busulfan: GSTA1 genotype is not a predictive factor of initial dose in Chinese adult patients undergoing hematopoietic stem cell transplantation
Cancer Chemotherapy and Pharmacology (2020)
-
Monitoring of Busulphan Concentrations in Children Undergone Hematopoietic Stem Cell Transplantation: Unicentric Experience over 10 years
European Journal of Drug Metabolism and Pharmacokinetics (2018)
-
Optimizing drug therapy in pediatric SCT: Focus on pharmacokinetics
Bone Marrow Transplantation (2015)
-
Allogeneic hematopoietic SCT for adults AML using i.v. BU in the conditioning regimen: outcomes and risk factors for the occurrence of hepatic sinusoidal obstructive syndrome
Bone Marrow Transplantation (2014)