Abstract
Pancreatic ductal adenocarcinoma has a median survival of less than 6 months from diagnosis. This is due to the difficulty in early diagnosis, the aggressive biological behaviour of the tumour and a lack of effective therapies for advanced disease. Mammalian heparanase is a heparan-sulphate proteoglycan cleaving enzyme. It helps to degrade the extracellular matrix and basement membranes and is involved in angiogenesis. Degradation of extracellular matrix and basement membranes as well as angiogenesis are key conditions for tumour cell spreading. Therefore, we have analysed the expression of heparanase in human pancreatic cancer tissue and cell lines. Heparanase is expressed in cell lines derived from primary tumours as well as from metastatic sites. By immunohistochemical analysis, it is preferentially expressed at the invading edge of a tumour at both metastatic and primary tumour sites. There is a trend towards heparanase expression in metastasising tumours as compared to locally growing tumours. Postoperative survival correlates inversely with heparanase expression of the tumour reflected by a median survival of 34 and 17 month for heparanase negative and positive tumours, respectively. Our results suggest, that heparanase promotes cancer cell invasion in pancreatic carcinoma and could be used as a prognostic indicator for postoperative survival of patients.
Similar content being viewed by others
Main
PDA has a poor prognosis due to its aggressive biological behaviour. In the majority of cases, diagnosis is only established late in the course of the disease, when local invasive growth and distant metastasis have already occurred (Baumel et al, 1994; Murr et al, 1994; Robert Koch Institut, 2002). At that point, the opportunity for curative therapy, which is surgery at an early stage, has already passed. Even though complete resection may still be achieved, the rate of relapses is high (Connolly et al, 1987; Trede et al, 1990; Cameron et al, 1991). Adjuvant combination therapy may offer a small survival advantage but is associated with treatment related morbidity. It therefore is not unequivocally accepted (Kalser and Ellenberg, 1985; Ahlgren, 1996; Jeekel, 1997; Klinkenbijl et al, 1999). The cytotoxic agent gemcitabine is the only drug currently known to be effective for palliation. Nevertheless, the absolute gain in survival is marginal (Burris et al, 1997). A number of markers have been associated with therapy-resistant tumours or shorter survival. Their validity as prognostic criterion remains to be confirmed, though (Compton et al, 1997).
On the molecular level, several events including proto-oncogene mutations such as K-ras (Almoguera et al, 1988), tumour suppressor gene mutations in the p16INK4a, DPC4, and p53 genes (Berrozpe et al, 1994; Caldas et al, 1994; Redston et al, 1994; Schutte et al, 1997; Goggins et al, 1998), and overexpression of a variety of growth factors and their receptors (Korc, 1998) have been described in the carcinogenesis of pancreatic cancer. The underlying causes for the aggressiveness of this cancer are still not fully understood.
Invasion of tumour cells into the surrounding tissue requires loosening of cell–cell adhesion, invasion of the basement membrane and disassembly of the extracellular matrix. Chief components of basement membrane (BM) and extracellular matrix (ECM) such as collagens, fibronectin and laminin are substrates of metalloproteinases and cysteine and serine proteases, which are known to be upregulated in metastatic cancers (Chambers and Matrisian, 1997).
Recently, another ECM degrading enzyme, mammalian heparanase, has been cloned from human placenta tissue and platelets and a putative 65 kDa precursor and a 50 kDa recombinant active enzyme have been expressed. Preliminary results suggest that heparanase may be a cell membrane protein (Hulett et al, 1999; Vlodavsky et al, 1999). The enzyme cleaves another key component of BM and ECM, namely heparan sulphate proteoglycans (HSPG) which belong to the glycosaminoglycans. Heparanase expression and function may be regulated at the transcriptional and posttranscriptional level (Gilat et al, 1995; Andela et al, 2000), though this topic awaits further clarification.
In addition to the enzymatic degradation of the ECM heparanase acts by releasing growth factors such as bFGF, heparan sulphate (HS) fragments and other enzymes such as lipoprotein lipase from the ECM. Consistent with its molecular capabilities heparanase has been shown to stimulate wound healing and tumour angiogenesis in vivo (Elkin et al, 2001). Its expression is upregulated in a number of metastatic tumour cell lines and transfection of tumour cells with heparanase cDNA conferred a highly metastatic phenotype (Hulett et al, 1999; Vlodavsky et al, 1999).
Heparanase expression has been studied in human primary tumours and tumour cell lines. Its expression correlates with tumour progression and invasiveness in colorectal and hepatocellular carcinoma (Friedmann et al, 2000; El-Assal et al, 2001). In this study, we examined the expression of heparanase protein and mRNA in human PDA cell lines and tumours. In addition, we studied the correlation of heparanase expression and stage of disease with postoperative survival in the intent to identify putative prognostic markers.
Materials and methods
Cell Culture and DNA transfection
Eight pancreatic cancer cell lines (AsPC-1, BxPC-3, Capan-1, Hs 766T, PANC-1, HPAF-II, CFPAC, MIA PaCa-2), the colorectal cancer cell line HT29 and the cervical cancer cell line HeLa were obtained from American Type Culture Collection (Manassas, USA) and maintained in the recommended growth conditions. Transfection for overexpression of Heparanase in HeLa cells was performed on cells growing at ∼70% confluence in T75 plates, using full-length Heparanase cDNA in pcDNA3 (Vlodavsky et al, 1999) and FuGENE 6 transfection reagent (Roche, Germany) according to the manufacturer's instruction.
RNA-isolation and semiquantitative RT–PCR
RNA was isolated from cell lines with the RNeasy Kit (Qiagen, Germany) according to the manufacturer's instructions. After reverse transcription of 5 μg total RNA by oligo(dT) priming (Superscript, Invitrogen, USA), the resulting cDNA was amplified by PCR using Taq DNA polymerase (Qiagen, Germany) and the heparanase primers 5′-GTGATGAGGCAAGTATTCTTTGG and 5′-TC AGATGCAAGCAGCAACTTTG. The PCR conditions were an initial denaturation of 5 min at 94°C and subsequent denaturation for 30 s at 94°C, annealing for 30 s at 58°C and extension for 1 min at 72°C (26 cycles). Standard β-actin primers and identical PCR conditions were used as internal input controls. PCR products were separated by 1.5% agarose gel electrophoresis and visualised by SYBR Green staining (Biozym Diagnostik, Germany).
Western blot analysis
Cells were dissolved in lysis buffer containing 5 M NaCl, 10% NP-40 (Igepal CA-630, Sigma-Aldrich, Germany), 1 M TRIS pH 8 and protease inhibitors (Complete Mini, EDTA free, Roche, Germany) at 4°C for 45 min and centrifuged at 13 000 r.p.m. for 20 min. The protein concentration of the supernatant was measured by the Bradford assay (BioRad, USA). 50 μg of each protein was separated by electrophoresis on 10% SDS/polyacrylamide gels and transferred to Hybond-P-Membrane (Amersham Pharmacia Biotech, UK) by semidry electroblotting (Transblot, BioRad, USA). Western blot analysis was carried out with a mouse monoclonal anti-heparanase antibody as previously described (Vlodavsky et al, 1999). A monoclonal antibody against β-actin (Sigma-Aldrich, USA) was used to verify equal loading of western blots. The secondary antibody used was a horseradish-peroxidase-conjugated goat-anti-mouse antibody (Pierce, USA). Immunoreactive bands were detected by enhanced chemiluminescence (SuperSignal, Pierce, USA) and exposure in a luminescent image analyser (LAS-1000plus, FujiFilm, USA).
Patients and tissue samples
Tissue specimens were chosen from 25 patients with chronic pancreatitis, from 50 patients with ductal adenocarcinoma of the pancreas undergoing surgery of curative intent between 1995 and 1999 and from five cases with normal pancreatic tissue. The PDA patients included 26 patients, in whom the tumours were R0 resected. Each tumour was re-evaluated with regard to typing, staging and grading. Tumour typing and staging were performed using WHO (Klöppel et al, 2000) and UICC (Sobin and Wittekind, 1997) criteria.
Immunohistochemistry
2–4 μm sections of formaline-fixed and paraffin-embedded specimens were deparaffinised and rehydrated. Endogenous peroxidase was blocked by 3% H2O2. Tissue was then demethylated in a microwave oven, incubated with citrate buffer, and blocked with goat serum. Sections were incubated with the monoclonal anti human heparanase antibody (Vlodavsky et al, 1999) or with non-immune mouse serum for negative control, respectively. Following washes with PBS sections were incubated with biotinylated goat anti-mouse IgG+IgM antibodies (BioGenex, USA), rinsed with PBS and incubated with peroxidase-labelled streptavidin. Colour was developed using Sigma Fast 3,3′-Diaminobenzidine (Sigma-Aldrich, USA). Counterstaining was performed with Mayer's hematoxylin. Samples were evaluated by microscopy and semiquantitavely analysed for heparanase expression (intense, moderate or absent staining).
Riboprobe preparation and in situ hybridisation
A 482 bp fragment of the human heparanase cDNA (primers identical to RT–PCR primers) was subcloned into the pGEMT vector multiple cloning site (Promega, USA). The linearized vector was used as a template for in vitro transcription and digoxigenin labeling of antisense or sense riboprobe using SP6 and T7-RNA polymerase (Roche, Germany). In situ hybridisation was performed as described before (Vlodavsky et al, 1999; Friedmann et al, 2000). Briefly, 5 μm sections were dewaxed and rehydrated, denaturated with 0.2 N HCl for 10 min and then digested with proteinase K (200 μg ml−1) at 37°C for 30 min. Digestion was stopped with two changes of H2O. Slides were prehybridised and hybridised as described (Vlodavsky et al, 1999; Friedmann et al, 2000). Probe concentration was 2 μg ml−1. Washes after hybridisation, incubation with anti-digoxigenin antibodies, and calorimetric detection were performed as described before (Vlodavsky et al, 1999; Friedmann et al, 2000). Some of the slides were counterstained with Mayer's hematoxylin.
Statistical analysis
Fisher's exact test was used to calculate the significance levels of contingency tables for differences of Heparanse expression regarding UICC stage, lymph node metastasis, distant metastasis or grading. For patients with R0 resected tumours postoperative survival periods were computed by the Kaplan–Meier method and compared by the Log Rank test. A P-value <0.05 was considered significant. SPSS-statistical software (SPSS), version 10, was used for data analysis.
Results
Heparanase expression in human pancreatic cancer cell lines
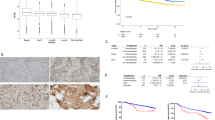
We examined eight human pancreatic cancer cell lines from different tissues of origin for the expression of heparanase mRNA and protein. Four of the cell lines (CFPAC, AsPC-1, HPAF-II, Hs 766T) had been established from distant metastases (liver, ascites) and lymph node metastases, respectively. Heparanase was expressed in all cell lines, but could hardly be detected in two cell lines (BxPC-3, Hs 766T) as determined by Western blot (Figure 1A). The level of expression varied widely. Apparently, heparanase expression did not correlate with whether or not the cell line was derived from a metastatic site. We also looked for levels of mRNA expression by quantitative RT–PCR. Levels of mRNA correlated closely with protein expression in the tumour cell lines examined (Figure 1B).
(A) Western blot analysis of heparanase expression in the human pancreatic cancer cell lines (AsPC-1 [4], BxPC-3 [5], Capan-1 [6], CFPAC [7], HPAF-II [8], Hs 766T [9], MIA PaCa-2 [10] and PANC-1 [11]). The colorectal cancer cell line HT-29 [1] and the cervical cancer cell line HeLa transfected with heparanase cDNA were used as positive controls. Untransfected HeLa cells were used as negative control [3]. β-Actin Western blot to confirm equal protein loading. (B) Semiquantitative RT–PCR of heparanase mRNA in human pancreatic cancer cell lines. DNA ladder [1], water control [2], AsPC-1 [3], BxPC-3 [4], Capan-1 [5], CFPAC [6], HPAF-II [7], Hs 766T [8], MIA PaCa-2 [9] and PANC-1 [10].
Heparanase expression in human pancreatic cancer tissue
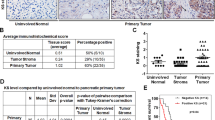
We analysed sections of tumour tissue from 50 patients (mean age 63 years, range 41–77 years, 27 male/23 female) that were operated on for pancreatic cancer and from 25 patients with chronic pancreatitis. Of the 50 tumour patients, 26 underwent a potentially curative resection, whereas the remaining 24 had microscopic evidence of non-curative resection (R1 resection). By immunohistochemical analysis, there was no detectable heparanase expression in normal pancreatic tissue. In contrast, heparanase showed high, moderate, or no expression in 17, 22, and 11 tumour specimens, respectively. Heparanase was preferentially expressed at the invading edge of PDA, though there was also some level of expression in the surrounding inflamed tissue (Figure 2). The level of expression was higher in primary tumours than at metastatic sites. Heparanase was shown to be moderately expressed in chronic pancreatitis. In situ hybridisation demonstrated, that the mRNA expression pattern followed closely that of protein expression.
Heparanase expression and localisation in primary human pancreatic cancers (A,D,G), chronic pancreatitis (B,E) and histologically normal pancreas (C,F). HE staining (A,B,C) for histological evaluation. Immunohistochemical staining with a monoclonal α-heparanase Ab and in situ hybridisation with an antisense (G) and sense probe (H, negative control). Normal pancreas with absent (F) and chronic pancreatitis with moderate (E) heparanase expression. Carcinoma tissue with strong heparanase expression (D,G), or absent heparanase expression (M). Heparanase positive tumour cells invading lymph sheets (I). Lymph node metastasis with strong (K) or absent heparanase expression (I).
There was no correlation between UICC stage of disease and heparanase expression. In contrast, there was a trend towards heparanase expression in lymph node positive vs negative tumours (P=0.064) (Table 1 and Figure 3).
Heparanase expression and postoperative survival of patients
In the intent to determine the prognostic value of heparanase in pancreatic cancer, we have analysed heparanase expression, stage of disease and survival of those 26 patients, that had been curatively resected (R0 resection). There was a significant negative correlation between postoperative survival and heparanase expression (Figure 4) (P<0.01). Mean postoperative survival was 34 months and 17 month in heparanase negative and positive tumours (R0 resection), respectively. Survival of patients with tumours expressing high or moderate levels of heparanase did not differ significantly. Multivariate analysis could not establish heparanase expression as an independent prognostic factor due to a lack of statistical power. There was no correlation between UICC stage of disease and postoperative survival in this series (data not shown).
Survival curves of 26 pancreatic cancer patients with R0 resection. Kaplan–Meier plots of postoperative survival curves of patients with or without heparanase expression in the primary carcinoma. Log-rank analysis proves a shorter postoperative survival period for patients with heparanase expression in their tumours (P<0.01).
Discussion
PDA is characterised by its local invasive growth including invasion of the perineurium and by a high frequency of metastases. In our study, heparanase was expressed in the majority of tumour specimens and expression at the invading edge of tumours could be demonstrated. This finding underscores the potential role of heparanase in tumour invasion and may partly explain the aggressiveness of PDA. We could confirm previous results that heparanase expression levels are higher at the primary tumour site than at metastatic sites (Koliopanos et al, 2001). This possibly reflects the need to break down physiological restraints like BM and ECM before a tumour cell can metastasise. How expression of heparanase is downregulated once a cell has settled at its metastatic site is still unresolved. In light of the results of other studies of heparanase expression in various tumours (Friedmann et al, 2000; El-Assal et al, 2001; Ginath et al, 2001) heparanase activation appears to be a crucial step for a tumour to become systemic.
Therapeutic options in PDA are scarce. There is no established adjuvant therapy after successfull operation despite the fact that the disease frequently relapses. Palliative treatments apart from best supportive care offer only marginal improvements in quality of life and survival, if any (Ahlgren, 1996; Burris et al, 1997; Jeekel, 1997). Therefore, it would be helpful to identify patients that might benefit from adjuvant therapy. We found that patients with heparanase expressing PDA had a significantly shorter postoperative survival than patients with no heparanase expression. In contrast, neither stage of disease nor lymph node involvement predicted prognosis, although this may be due to the small study size. Our finding is in agreement with previously published results (Koliopanos et al, 2001). We have to caution, though, that the number of patients in our study precluded a multivariate analysis. Prospective studies could lend further support to our finding. The relatively long postoperative survival periods in our study are probably due to a positive selection of patients whose tumours were deemed to be resectable preoperatively.
An important question is how heparanase expression and function are regulated under different physiological and pathophysiological conditions. Clues to physiological mechanisms may be infered from the role of heparanase in pregnancy, morphogenesis and development (Dempsey et al, 2000; Goldshmidt et al, 2001). Heparanase expression is also evident in inflammation, angiogenesis and cancer metastasis (Vlodavsky and Friedmann, 2001). Heparanase is expressed by a variety of cell types and helps the cells to cross physiological boundaries, as is the case with trophoblasts in the placenta, leukocyte extravasation, or tumour cell metastasis (Parish et al, 2001). There are as yet few identified molecular mechanisms of heparanase regulation. These comprise transcriptional and posttranscriptional mechanisms such as tissue-pH and regulation by NF-κB and proto-oncogenes (Schwarz et al, 1990; Gilat et al, 1995; Andela et al, 2000). In our study expression of heparanase mRNA and protein in vivo showed significant overlap. Transcriptional upregulation therefore appears to be the main way of PDA cells to benefit from the prometastatic and angiogenic effects of heparanase.
The key role of heparanase in tumorigenesis and the existing evidence for only one endogenous mammalian heparan sulphate degrading endoglycosidase (Hulett et al, 1999; Vlodavsky et al, 1999) has spurred efforts to develop therapeutic applications. So far, non-anticoagulant heparins and sulphated oligosaccharides show promising results in animal studies of tumour spreading (Miao et al, 1999; Parish et al, 1999). A drug of the latter group, Phosphomannopentaose sulfate (PI-88), slowed tumour growth and metastasis and reduced the vascularity of tumours both in vitro and in vivo in animals (Parish et al, 1999). Hopefully, this and other studies will emerge into clinical trials of the most promising of these drugs.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahlgren JD (1996) Chemotherapy for pancreatic carcinoma. Cancer 78: 654–663
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53: 549–554
Andela VB, Schwarz EM, Puzas JE, O'Keefe RJ, Rosier RN (2000) Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappa B. Cancer Res 60: 6557–6562
Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H (1994) Results of resection of cancer of the exocrine pancreas: a study from the French association of surgery. Br J Surgery 81: 102–107
Berrozpe G, Schaeffer J, Peinado MA, Reak FS, Perucho M (1994) Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer 58: 185–191
Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvement in survival and clinical benefit with gemcitabine as first line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413
Caldas C, Hahn SA, daCosta LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE (1994) Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in PDA. Nature Genet 8: 27–31
Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J (1991) Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 161: 120–125
Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260–1270
Compton CC, Mulvihill SJ (1997) Prognostic factors in pancreatic carcinoma. Surg Oncol Clin N Am 6: 533–554
Connolly MM, Dawson PJ, Michelassi F, Moosa AR, Lowenstein F (1987) Survival in 1001 patients with carcinoma of the pancreas. Ann Surg 206: 366–373
Dempsey LA, Brunn GJ, Platt JL (2000) Heparanase, a potent regulator of cell matrix interactions. Trends Biochem Sci 25: 349–355
El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N (2001) The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res 7: 1299–1305
Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I (2001) Heparanase as mediator of angiogenesis: mode of action. FASEB J 15: 1661–1663
Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, Pecker I, Pappo O (2000) Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Am J Pathol 157: 1167–1175
Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O (1995) Molecular behaviour adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH. J Exp Med 181: 1929–1934
Ginath S, Menczer J, Friedmann Y, Aingorn H, Aviv A, Tajima K, Dantes A, Glezerman M, Vlodavsky I, Amsterdam A (2001) Expression of heparanase, Mdm2, and erbB2 in ovarian cancer. Int J Oncol 18: 1133–1144
Goggins M, Sheker M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE (1998) Genetic alterations of the transforming growth factor β receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res 58: 5329–5332
Goldshmidt O, Zcharia E, Aingorn H, Guatta-Rangini Z, Atzmon R, Michal I, Pecker I, Mitrani E, Vlodavsky I (2001) Expression pattern and secretion of human and chicken heparanase are determined by their signal peptide sequence. J Biol Chem 276: 29178–29187
Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR (1999) Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nature Med 5: 803–809
Jeekel J (1997) Adjuvant or neoadjuvant therapy for pancreatic carcinoma?. Digestion 58: 533–535
Kalser MH, Ellenberg SS (1985) Pancreatic cancer. Adjuvant combined radiation and chemotherapy following resection. Arch Surg 120: 899–903
Klinkenbijl JH, Jeekel J, Sahmoud H, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230: 776–782
Klöppel K, Hruban RH, Longnecker DS, Adler G, Kern SE, Partanen TJ (2000) Ductal adenocarcinoma of the pancreas. In Pathology and Genetics of Tumours of the Digestive System. WHO Classification of Tumours Hamilton SR, Aaltonen LA (eds) Lyon: IARC Press
Koliopanos A, Friess H, Kleeff J, Shi X, Liao Q, Pecker I, Vlodavsky I, Zimmermann A, Büchler MW (2001) Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res 61: 4655–4659
Korc M (1998) Role of polypeptide growth factors and their receptors in human pancreatic cancer. In Pancreatic cancer: Pathogenesis, diagnosis, and treatment Reber HA (ed) pp 21–32, Totowa, NJ: Humana Press
Miao HQ, Elkin M, Aingorn E, Ishai-Michaeli R, Stein CA, Vlodavsky I (1999) Inhibition of heparanase activity and tumor metastasios by laminarin sulfate and synthetic phophorothioate oligodeoxynucleotides. Int J Cancer 83: 424–431
Murr MM, Sarr MG, Oishi AJ, van Heerden JA (1994) Pancreatic cancer. CA Cancer J Clin 446: 304–318
Parish CR, Freeman C, Brown KJ, Francis DJ, Cowden WB (1999) Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase assays. Cancer Res 59: 3433–3441
Parish CR, Freeman C, Hulett MD (2001) Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta 1471: M99–M108
Redston MS, Caldas C, Seymour AB, Hruban RH, daCosta L, Yeo CJ, Kern SE (1994) p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res 54: 3025–3033
Robert Koch Institut (2002) http://www.rki.de/CHRON/KREBS/KREBS.HTM
Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W, Baylin SB, Kern SE, Herman JG (1997) Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 57: 3126–3130
Schwarz LC, Inoue T, Irimura T, Damen JE, Greenberg AH, Wright JA (1990) Relationship between heparanase activity and increasing metastatic potential of fibroblasts transfected with various oncogenes. Cancer Lett 51: 187–192
Sobin LH, Wittekind C (1997) International Union Against Cancer TNM classification of malignant tumours. New York: Wiley-Liss Press
Trede M, Schwall G, Saeger HD (1990) Survival after pancreatoduodenectomy. Ann Surg 211: 447–458
Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I (1999) Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nature Med 5: 793–802
Vlodavsky I, Friedmann Y (2001) Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest 108: 341–347
Acknowledgements
The authors are very grateful to Professor Israel Vlodavsky (Department of Oncology, Hadassah-Hebrew University Hospital, Jerusalem, Israel) for providing us with the antibody and critically reading the manuscript. We thank Martina Fügenschuh and Mandy Klaske, Leipzig University, for their excellent technical assistance. This study was partially supported by a grant (CA 207/3-1) from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rohloff, J., Zinke, J., Schoppmeyer, K. et al. Heparanase expression is a prognostic indicator for postoperative survival in pancreatic adenocarcinoma. Br J Cancer 86, 1270–1275 (2002). https://doi.org/10.1038/sj.bjc.6600232
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600232
Keywords
This article is cited by
-
Heparanase and the hallmarks of cancer
Journal of Translational Medicine (2020)
-
Stellenwert von prognostischen Biomarkern in der offenen und endovaskulären Aortenchirurgie
Gefässchirurgie (2018)
-
Clinicopathological and prognostic significance of myofibrillogenesis regulator-1 protein expression in pancreatic ductal adenocarcinoma
Tumor Biology (2013)
-
Heparanase enzyme in chronic inflammatory bowel disease and colon cancer
Cellular and Molecular Life Sciences (2012)