Abstract
GTP-cyclohydrolase deficiency in dopa-responsive dystonia (DRD) patients impairs the biosynthesis of dopamine, but also of serotonin. The high prevalence of non-motor symptoms suggests involvement of the serotonergic pathway. Our study aimed to investigate the serotonergic system in vivo in the brain of`DRD patients and correlate this to (non-)motor symptoms. Dynamic [11C]DASB PET scans, a marker of serotonin transporter availability, were performed. Ten DRD, 14 cervical dystonia patients and 12 controls were included. Univariate- and network-analysis did not show differences in binding between DRD patients compared to controls. Sleep disturbances were correlated with binding in the dorsal raphe nucleus (all participants: rs = 0.45, p = 0.04; patients: rs = 0.64, p = 0.05) and participants with a psychiatric disorder had a lower binding in the hippocampus (all participants: p = 0.00; patients: p = 0.06). Post-hoc analysis with correction for psychiatric co-morbidity showed a significant difference in binding in the hippocampus between DRD patients and controls (p = 0.00). This suggests that psychiatric symptoms might mask the altered serotonergic metabolism in DRD patients, but definite conclusions are difficult as psychiatry is considered part of the phenotype. We hypothesize that an imbalance between different neurotransmitter systems is responsible for the non-motor symptoms, and further research investigating multiple neurotransmitters and psychiatry in DRD is necessary.
Similar content being viewed by others
Introduction
Dopa-responsive dystonia (DRD) is a rare inherited metabolic disorder in which patients present with dystonia characterized by diurnal fluctuations and an excellent response to levodopa treatment1. The most common type of dopa-responsive dystonia is caused by an autosomal dominant inherited mutation in the GCH1 gene (OMIM#600225) affecting the enzyme GTP cyclohydrolase 12. This enzyme is the first and rate-limiting step in the biosynthesis of tetrahydrobiopterin (BH4), which is an essential co-factor involved in the biosynthesis of mono-amine neurotransmitters, such as dopamine and serotonin.
The biochemical changes in dopamine are considered to be responsible for the motor phenotype of dystonia and Parkinsonism in DRD patients and hence the excellent response to levodopa therapy. Besides these motor manifestations, attention has been drawn to non-motor symptoms in DRD with an increased prevalence of psychiatric and sleep disorders compared to controls3,4,5. The pathophysiology of non-motor symptoms in dystonia is not known, however, serotonin is suggested to play an important role1,6. As already indicated, the biosynthesis of serotonin, identical to that of dopamine, is impaired due to the GCH1 mutation and subsequent deficiency of BH4. In line with this, decreased levels of 5-Hydroxyindoleacetic acid (5-HIAA), the final breakdown product of serotonin, in cerebral spinal fluid of DRD patients has been described3,7. Furthermore, the serotonergic system is important in the regulation of mood and sleep, and is implicated in the pathophysiology of many psychiatric disorders.
To investigate the potential role of the serotonergic system in the brain of DRD patients, positron emission tomography (PET) can be used. The radiotracer [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)benzonitrile ([11C]DASB) is a well-validated and selective radio-ligand that binds to the serotonin reuptake transporter (SERT) and qualifies as an excellent biomarker to study the serotonergic system in vivo8.
Dystonia, including DRD, is considered to be a network disorder, with multiple brain regions involved in its pathophysiology9. Therefore, a network-based analysis may provide useful additional information. A frequently used method to create disease-specific patterns is the scaled subprofile model, which is a spatial covariance method based on principal component analysis (SSM/PCA)10. Recently, a disease-specific covariance pattern on [11C]DASB PET scans was detected using this method in Parkinson’s disease11.
To the best of our knowledge, there are no published studies investigating SERT status in vivo in DRD patients using [11C]DASB PET scans. Therefore, this study aimed to examine group differences in serotonergic system integrity between controls and DRD patients using both univariate- and network-based analysis. The second aim is to investigate the relationship between (non-)motor symptoms and the serotonergic system in the brain of DRD patients.
Materials and methods
Participants and clinical measures
Ten DRD patients with a confirmed mutation in the GCH1 gene were included. Data of two control groups, of which data was published before12, were used: (1) 14 clinically diagnosed idiopathic cervical dystonia (CD) patients, (2) 12 controls without a movement disorder. No pilot PET data on SERT receptor availability in patients with dystonia was available, therefore, we based our sample size on previous studies on SERT status in (neuro)psychiatric disorders which used 10–15 participants13,14. None of the participants were using serotonergic medication that influences serotonin, including antidepressants, or had relevant neurological comorbidity.
Clinical and demographic characteristics of the patients were obtained through a standardized interview. The severity of dystonia was assessed with the Burke Fahn Marsden Dystonia Rating Scale (BFMDRS) and the Clinical Global Impression Scale (CGI). Non-motor symptoms were assessed using validated questionnaires. The Mini International Neuropsychiatric Interview—PLUS (MINI-PLUS) was used to evaluate the presence of psychiatric disorders. Severity of depression, anxiety, fatigue, excessive daytime sleepiness and quality of sleep were assessed with the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), the Fatigue Severity Scale (FSS), the Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index (PSQI), respectively.
Written informed consent was obtained from all subjects according to the Declaration of Helsinki. This study was approved by the medical ethical committee of the UMCG (METc 034/14).
SERT polymorphism
Three different alleles in the SERT-linked polymorphic region (5-HTTLPR) are known to be associated with different transcriptional activity and, therefore, influence the number of expressed SERT15. A set of specific single-nucleotide polymorphisms in the 5-HTTLPR was tested as described before12.
Scanning protocol
Individual axial 3D T1-weighted gradient-echo images (3T Intera, Philips, The Netherlands) of the brain were acquired from all participants. PET imaging of DRD patients was performed using the same protocol as previously published for CD patients and controls with either a Biograph 40-mCT or 64-mCT (Siemens Healthcare, USA). The protocol consisted of a 60-min dynamic acquisition scan starting simultaneously with an intravenous bolus of [11C]DASB (mean dose 382 ± 41 MBq)16. PET images were reconstructed from list-mode data into 23 frames (7 × 10 s, 2 × 30 s, 3 × 1 min, 2 × 2 min, 2 × 3 min, 5 × 5 min, and 2 × 10 min). Head movement was minimized with a head-restraining band. DRD patients were allowed to continue using their levodopa treatment during the day of the scan.
Image processing and analysis
Reconstruction of the dynamic [11C]DASB images was performed using the 3D OSEM algorithm (3 iterations and 24 subsets), point spread function correction, and time-of-flight, resulting in a matrix of 400 × 400 × 111 of isotropic 2-mm voxels, smoothed with 2-mm filter at full width at half maximum (FWHM).
Image processing was performed with PMOD v3.8 software. We applied movement correction (frames 13–23, using 1–12 as reference) before the individual PET image was matched to the individual MRI. A six-tissue probability map was used to estimate grey and white matter volumes and a normalization of the individual MRI onto the Montreal Neurological Institute (MNI) standard space was calculated and applied to the corresponding PET image. Predefined volumes of interest (VOI) based on Hammers atlas were transformed into the individual MRI space17. As no differences between the left and right hemispheres were identified, a weighted average, based on the volume of the region, was calculated from the corresponding regions in both hemispheres. VOIs for the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN) were manually defined by selecting the 80% of the voxels with the highest uptake within a sphere according to visual inspection of the PET image, as described previously (subject-based: sDRN and sMRN)12. The DRN and MRN were also defined based on their MNI coordinates (atlas-based: aDRN and aMRN)18. A Gaussian kernel of 6-mm FWHM was applied before pharmacokinetic modeling was performed with PXMOD tool of PMOD V4.0 software. Non-displaceable binding potential (BPND) parametric maps were calculated using the Simplified Reference Tissue Model 2 (SRTM2) with the cerebellum (excluding vermis) as reference region and the striatum as high-binding region for estimation of k2′19.
For the VOI-based analysis, average BPND values per region in individual space were used. For both voxel-based analysis and SSM/PCA, BPND parametric maps were used in MNI space.
SSM/PCA analysis
SSM/PCA analysis was performed in order to assess whether a disease-specific pattern of BPND could discriminate DRD from CD and controls. SSM/PCA analysis was performed in MATLAB R2018b using in-house software which was validated against the original study where these mathematical principles are extensively reported20,21. In brief: BPND data was masked and centered twice, by subtracting subject and region means to obtain a matrix of subject residuals. A covariance matrix was constructed of these residuals and PCA was applied, resulting in principle components (PCs). The PCs that cumulatively account for 50% of the variance were selected. A stepwise forward combination was used to generate the disease patterns and the pattern with the lowest Akaike information criteria was selected as the final disease pattern. A score per subject was calculated by taking the inner product of the disease pattern and the subject’s image. To robustly test the generated scores, a leave-one-out cross-validation (LOOCV) was applied. A receiver operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated to assess the discriminating values of the pattern.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 23. All baseline data were quantitatively described. Pearson’s χ2 test, one-way ANOVA, Mann Whitney U or Kruskall Wallis tests were used to compare the demographic and clinical data between groups.
For the VOI based analysis, BPND values were compared between respectively the DRD and CD and DRD and healthy control group with a Mann Whitney U test. Correction for multiple comparisons (Bonferroni) was performed and the statistical significance threshold was set at a p-value < 0.004 (= 0.05/13 VOIs). An ANCOVA, after a square root transformation of the BPND values, was performed to correct for presence of psychiatric co-morbidity.
Voxel-based analysis was performed with SPM12 (Wellcome Trust Centre for Neuroimaging, UK) and a two-sample t-test was used to assess differences between DRD patients and CD patients, and DRD patients and healthy controls. T-maps data were interrogated at p = 0.005 (uncorrected) and only clusters with p < 0.05 corrected for family-wise error were considered significant.
Spearman’s rho test corrected for multiple testing (Benjamini–Hochberg) was used to perform a correlation analysis between the BPND in the VOIs and clinical variables in all participants. To assess differences in BPND between participants with a lifetime psychiatric diagnosis and those without, a Mann Whitney U test was performed. To determine whether the significant correlations that we found in the whole group were also present in the DRD group, above mentioned tests were also performed in the DRD group.
Finally, to check for possible confounders, a Spearman’s rho test was performed between dosage of levodopa, age, La/La genotype and BPND.
Ethics approval
This study was approved by the medical ethical committee of the UMCG (METc 034/14).
Consent to participate
Written informed consent was obtained from all subjects according to the Declaration of Helsinki.
Results
Demographic information and clinical characteristics can be found in Table 1. The median age of DRD patients was not significantly different compared to the control groups and no differences in smoking habit or La/La genotype were detected. Eight DRD patients were using levodopa (median daily dose of levodopa 150 mg, range 100–400 mg). One of the remaining two patients, who weren’t using levodopa, was an asymptomatic mutation carrier, and the other patient reported to have no symptoms but based on neurological examination did have mild dystonia (BFMDRS 9.5). No other dopaminergic drugs were used. Dystonia was well controlled in the DRD group with a median BFMDRS of 6.5 (max. score of 120), and a median CGI score of 2 (max. score of 7) which was significantly lower than in the CD group (median 4.5). No symptoms of Parkinsonism were observed in DRD group.
Psychiatric co-morbidity was highly present in both dystonia groups (DRD 60%, CD 79%) compared to the controls (33%). The severity scores assessing severity of depression, anxiety, sleep problems and fatigue were also significantly different between the groups, with more severe non-motor symptoms in both dystonia groups (Table 1).
VOI and voxel-based analysis
Visual assessment of the [11C]DASB PET scans revealed high BPND in the dorsal midbrain, thalamus, and striatum in all groups (Fig. 1).
Non-displaceable binding potential (BPND). Example of the non-displaceable binding potential of [11C]DASB in a dopa-responsive dystonia (DRD) patient, a cervical dystonia (CD) patient and a control participant without a movement disorder. The DRD patient had dystonia for 43 years, was diagnosed with a panic disorder and had sleep disturbances; the CD patient suffered from dystonia for 52 years, was diagnosed with depression and panic disorder and was easily fatigued, the healthy control had no history of a psychiatric disorder and or sleep disturbance.
Further analysis showed no significant differences in the BPND of VOIs between the DRD and CD group, nor between the DRD and the healthy control group (Fig. 2). The mean volume of the VOIs was not statistically different between groups (Supplementary Table 1).
Accordingly, voxel-based analysis did not show any significant differences between the DRD and the two control groups.
SSM/PCA analysis
No disease-related covariance pattern could be created with SSM/PCA method that discriminates DRD from CD patients. We did create a disease-related covariance pattern comparing DRD patients to controls without a movement disorder. However, the subject scores were not significantly different between the groups (DRD vs. healthy controls: median 1042 vs. 3839, p = 0.77) and the AUC of the ROC curve was 0.48, indicating that the pattern did not effectively discriminate between the two groups (Fig. 3).
SSM/PCA analysis subject score and relative operating characteristic curve. Left: subject scores for the disease related covariate pattern after a leave-one-out cross-validation (LOOCV). Right: relative operating characteristic (ROC) curve for the disease-related covariate pattern after LOOCV with an area under the curve (AUC) of 0.5.
Relationship (non-)motor symptoms and BPND
No significant correlations between severity of the dystonia (CGI) and BPND in any of the VOIs was found in all participants nor in the DRD group (Supplementary Fig. 1).
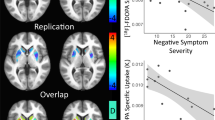
In all participants a significant association was found between quality of sleep and BPND in the sDRN (rs = 0.45, p = 0.04), indicating a higher BPND in participants with a worse quality of sleep. This positive association was also present in the DRD patients, this almost reached statistical significance after correction for multiple comparisons (rs = 0.64, p = 0.05) (Fig. 4). In DRD patients, a negative association between fatigue and the BPND in the sMRN (rs = − 0.66, p = 0.05) was also found, indicating a lower BPND in patients with more fatigue. This finding was not present in the whole group.
Associations between non-motor symptoms and non-displaceable binding potential (BPND). (A) Correlation, calculated with Spearman’s rho test, between quality of sleep and BPND in dorsal raphe nucleus (sDRN) in all participants, p-value is corrected for multiple comparisons. (B) Correlation, calculated with Spearman’s rho test, between quality of sleep and BPND in sDRN in DRD patients, p-value is corrected for multiple comparisons. (C) Dotplot with median (line) of BPND in hippocampus and presence of a life time psychiatric diagnosis in all participants. (D) Dotplot with median (line) of BPND in hippocampus and presence of a life time psychiatric diagnosis in DRD patients. All results of the correlation analysis can be found in Supplementary Fig. S1.
Participants who were at least once in their life diagnosed with a psychiatric disorder had a lower BPND in the hippocampus (median 0.40 vs. 0.48, p = 0.00). In DRD patients this finding was also present, but did not reach statistical significance (median 0.36 vs. 0.53, p = 0.06).
Post hoc analysis
A post hoc analysis in which we corrected for the presence of a psychiatric disorder showed a significant difference in BPND in the hippocampus between DRD patients and respectively CD patients and healthy controls (uncorrected p-value = 0.004, Supplementary Table 2).
Confounders
In the DRD group no correlations between dosage of levodopa and BPND in any of the VOIs was found, and, visually, no differences in BPND were observed in the eight patients who were using levodopa versus the two who were not. No correlations between age and BPND in any of the VOIs were found in the whole group. The La/La genotype was associated with a lower BPND (rs = 0.48, p = 0.03) in the globus pallidus.
Discussion
This is the first in vivo study assessing the serotonergic brain metabolism in patients with GTP cyclohydrolase deficient DRD using [11C]DASB PET scans. Both the univariate- as well as the network analysis did not show any differences in SERT binding between DRD patients compared to CD patients or healthy controls. However, the highly prevalent non-motor symptoms did have an association with SERT binding; sleep disturbances and psychiatric co-morbidity were correlated with respectively the BPND in the raphe nuclei and hippocampus.
The deficiency of the enzyme GTP-cyclohydrolase 1 in patients with DRD leads to an impaired synthesis of BH4, resulting in a decreased synthesis of dopamine known to cause motor symptoms, while a decreased synthesis of serotonin hypothetically can be linked to the non-motor symptoms. However, we did not observe any differences in brain SERT binding in patients with DRD, suggesting that the number of SERT or their binding capacity was not different. Our finding is in line with a post-mortem case report that did not show altered levels of serotonin markers, including SERT protein in the striatum of a DRD patient22. On the other hand, low levels of 5-HIAA, one of the main downstream metabolites of serotonin, are reported in the CSF of some untreated DRD patients, which do suggest an altered serotonergic metabolism3,7. One important consideration is that with [11C]DASB PET scans we measured SERT, an important regulator of the synaptic concentration of serotonin, but we did not directly measure serotonin concentrations.
Another explanation for our negative findings could be that alterations in the serotonergic metabolism in DRD are too subtle to be detected with a single PET scan assessing only the binding to SERT. As we know that other neurotransmitters, such as dopamine and acetylcholine, are also involved in dystonia, it is very likely that the non-motor symptoms, in a similar way, can be the result of an imbalance between multiple neurotransmitters, rather than a disruption in the pathway of only serotonin. Other neurotransmitters such as dopamine, noradrenaline and acetylcholine are extensively studied with regard to psychiatric disorders and sleep disorders23,24,25 and are likely involved in non-motor symptoms in dystonia as well. Further studies assessing not only the serotonergic system, but combined with other neurotransmitter systems are necessary.
Assessing all participants we did, however, find an association between psychiatric co-morbidity and SERT binding in the hippocampus, indicating that the serotonergic system is involved in psychiatric disorders. A subsequently performed post-hoc analysis to correct for psychiatry in the VOI-based analysis revealed a significant difference in SERT binding in the hippocampus between DRD patients and the two control groups. This suggests that there might be an altered serotonergic system related to the motor symptoms in DRD patients, overshadowed by the high prevalence of psychiatric disorders and corresponding disturbances in the serotonergic system. This is a difficult concept as the psychiatric symptoms are currently considered part of the phenotype in DRD patients. Further studies in a larger group of DRD patients is required to further study the relationship of DRD patients with and without psychiatric symptoms with the serotonergic system.
Furthermore, the correlation analysis in all participants showed that a worse quality of sleep was associated with higher SERT binding in the DRN. This was also observed in the DRD group, though the correlation did not reach statistical significance after correction for multiple comparisons. In the past, several studies showed evidence of the complex interaction between the raphe nuclei and sleep26. Serotonin has a dual function in sleep and waking and this is now believed to depend, among other things, not only on the brain region and serotonin receptor type involved, but also on the interaction with other neurotransmitter systems27. This might explain the contrasting finding of an association between fatigue and a lower BPND in the median raphe nucleus in DRD patients. Again, the correlation was not statistically significant after correction for multiple comparisons (p = 0.05), probably due to the small sample size. Comparable results were found in two [11C]DASB studies in Parkinson’s disease, where patients with sleep dysfunction or fatigue had a lower BPND in several brain areas including the raphe nuclei as compared to patients without sleep problems28,29. Further studies assessing the role of serotonin in sleep and fatigue in dystonia patients are warranted to validate the findings of our study. Our results support that the serotonergic system is associated with both sleep and psychiatric disorders, but that this is not specific for DRD.
This study has several limitations that should be taken into consideration. The levodopa therapy that our patients were taking might have influenced our results. However, it was shown that levodopa therapy is able to reduce levels of serotonin and 5-HIAA in the brain even more30, so one would expect to find more differences in SERT binding. Furthermore, we did not find a correlation between dosage of levodopa and BPND in any of the VOIs in the DRD group. None of the participants were taking serotonergic medication during the study, however, one DRD patient used paroxetine 7 years prior to the scan, this might have had some influence on our results. Next, the number of participants was relatively small which is an inevitable reality when one investigates a rare disorder such as GTP cyclohydrolase deficient DRD, but this may account for our negative findings.
To conclude, no differences in SERT binding in DRD patients were found, indicating that either there is no altered serotonergic metabolism, or differences are too subtle to be detected with [11C]DASB PET scans alone. Associations between sleep problems, psychiatric diagnosis, and SERT binding were found in the whole group and were not specific for DRD. However, when corrected for psychiatric co-morbidity a significant difference in SERT binding in the hippocampus was found between the DRD patients and controls. This suggests an altered serotonergic metabolism in DRD, masked by the high prevalence of psychiatric disorders. Further research investigating psychiatric co-morbidity and multiple neurotransmitters, like dopamine, norepinephrine or acetylcholine together with serotonin, are necessary to shed light on the pathophysiology of the highly present non-motor symptoms in DRD.
Data availability
Raw data are available upon reasonable request, after signing a data use agreement of the UMCG.
Abbreviations
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- 5-HTTLPR:
-
Serotonin reuptake transporter-linked polymorphic region
- [11C]DASB:
-
[11C]-3-amino-4-(2-dimethylaminomethylphenylsulfanyl)benzonitrile
- BAI:
-
Beck Anxiety Index
- BDI:
-
Beck Depression Inventory
- BFMDRS:
-
Burke Fahn Marsden Dystonia Rating Scale
- BH4:
-
Tetrahydrobiopterin
- CD:
-
Cervical dystonia
- CGI:
-
Clinical Global Impression Scale
- DRD:
-
Dopa-responsive dystonia
- DRN:
-
Dorsal raphe nucleus
- ESS:
-
Epworth Sleepiness Scale
- FSS:
-
Fatigue Severity Scale
- MINI-PLUS:
-
Mini International Neuropsychiatric Interview—PLUS
- MRN:
-
Median raphe nucleus
- PSQI:
-
Pittsburgh Sleep Quality Index
- SSM/PCA:
-
Scaled subprofile model based on principal component analysis
- SERT:
-
Serotonin reuptake transporter
References
Wijemanne, S. & Jankovic, J. Dopa-responsive dystonia—Clinical and genetic heterogeneity. Nat. Rev. 11, 414–424 (2015).
Pagon, R. A. et al. (eds) GTP Cyclohydrolase 1-Deficient Dopa-Responsive Dystonia (University of Washington, 1993).
Van Hove, J. L. et al. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J. Neurol. Neurosurg. Psychiatry 77, 18–23 (2006).
Bruggemann, N. et al. Non-motor phenotype of dopa-responsive dystonia and quality of life assessment. Parkinsonism Relat. Disord. 20, 428–431 (2014).
Trender-Gerhard, I. et al. Autosomal-dominant GTPCH1-deficient DRD: Clinical characteristics and long-term outcome of 34 patients. J. Neurol. Neurosurg. Psychiatry 80, 839–845 (2009).
Smit, M. et al. Serotonergic perturbations in dystonia disorders—A systematic review. Neurosci. Biobehav. Rev. 65, 264–275 (2016).
Zambrino, C. A., Balottin, U., Borgatti, R. & Lanzi, G. Consideration on two cases of dystonia-parkinsonism. Ital. J. Neurol. Sci. 12, 475–478 (1991).
Kim, J. S., Ichise, M., Sangare, J. & Innis, R. B. PET imaging of serotonin transporters with [11C]DASB: Test-retest reproducibility using a multilinear reference tissue parametric imaging method. J. Nucl. Med. 47, 208–214 (2006).
Kojovic, M. et al. Secondary and primary dystonia: Pathophysiological differences. Brain 136, 2038–2049 (2013).
Eidelberg, D. Metabolic brain networks in neurodegenerative disorders: A functional imaging approach. Trends Neurosci. 32, 548–557 (2009).
Fu, J. F. et al. Investigation of serotonergic Parkinson’s disease-related covariance pattern using [(11)C]-DASB/PET. NeuroImage Clin. 19, 652–660 (2018).
Smit, M. et al. Relationships between serotonin transporter binding in the raphe nuclei, basal ganglia, and hippocampus with clinical symptoms in cervical dystonia: A [(11)C]DASB positron emission tomography study. Front. Neurol. 9, 88 (2018).
Maillet, A. et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 139, 2486–2502 (2016).
Nikolaus, S., Antke, C., Beu, M. & Müller, H.-W. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders—Results from in vivo imaging studies. Rev. Neurosci. 21, 119–139 (2010).
Nakamura, M., Ueno, S., Sano, A., Tanabe, H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry. 5(1), 32–38 (2000).
Stansley, B. J. & Yamamoto, B. K. L-dopa and brain serotonin system dysfunction. Toxics 3, 75–88 (2015).
Wilson, A. A. et al. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: Synthesis, radiosynthesis, and in vitro and ex vivo evaluation of 11C-labeled 2-(phenylthio)araalkylamines. J. Med. Chem. 43, 3103–3110 (2000).
Hammers, A. et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 19, 224–247 (2003).
Kranz, G. S., Hahn, A., Savli, M. & Lanzenberger, R. Challenges in the differentiation of midbrain raphe nuclei in neuroimaging research. Proc. Natl. Acad. Sci. U.S.A. 109, E2000–E2000 (2012).
Wu, Y. & Carson, R. E. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. Cereb. Blood Flow Metab. 22, 1440–1452 (2002).
Spetsieris, P. G., Ma, Y., Dhawan, V. & Eidelberg, D. Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. Neuroimage 45, 1241–1252 (2009).
Teune, L. K. et al. Validation of Parkinsonian disease-related metabolic brain patterns. Mov. Disord. 28, 547–551 (2013).
Furukawa, Y. et al. A marked contrast between serotonergic and dopaminergic changes in dopa-responsive dystonia. Neurology 87, 1060–1061 (2016).
Ressler, K. J. & Nemeroff, C. B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 12(Suppl 1), 2–19 (2000).
Scarr, E., Gibbons, A. S., Neo, J., Udawela, M. & Dean, B. Cholinergic connectivity: It’s implications for psychiatric disorders. Front. Cell. Neurosci. 7, 55 (2013).
Siegel, J. M. The neurotransmitters of sleep. J. Clin. Psychiatry 65(Suppl 1), 4–7 (2004).
Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 21, 24–27 (1999).
Ursin, R. Serotonin and sleep. Sleep Med. Rev. 6, 55–69 (2002).
Wilson, H., Giordano, B., Turkheimer, F. E., Chaudhuri, K. R. & Politis, M. Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. NeuroImage Clin. 18, 630–637 (2018).
Pavese, N., Metta, V., Bose, S. K., Chaudhuri, K. R. & Brooks, D. J. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133, 3434–3443 (2010).
Acknowledgements
The authors thank Dr. R.J. Renken for help with the SSM/PCA analysis and Dr. K.E. Niezen-Koning, I.H. Veen-Hof and A.J. Dijck-Brouwerd for determining the SERT polymorphism.
Funding
ET received funding from Junior Scientific Masterclass from the University of Groningen, University Medical Center Groningen. MS has received grants from the University Medical Center Groningen and Stichting Wetenschapsfonds Dystonie Vereniging. MT has received research grants from Stichting Wetenschapsfonds Dystonie Vereniging, Prinses Beatrix Foundation, Fonds NutsOhra, and unrestricted grants for DystonieNet from Ipsen Pharmaceuticals, Allergan Pharmaceuticals, Medtronic, and Actelion. The PET scans were funded by the University Medical Center Groningen—Healthy Ageing Pilot Fund. The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Author information
Authors and Affiliations
Contributions
Author contributions include conception and design (E.T., M.S., R.D., A.K., T.K., D.V.G., M.T.), data collection or acquisition (E.T., D.P., M.S., A.K., D.V.G.), statistical analysis (E.T., D.V.G.), interpretation of results (E.T., D.P., D.V.G., M.T.), drafting the manuscript work or revising it critically for important intellectual content (all authors) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timmers, E.R., Peretti, D.E., Smit, M. et al. Serotonergic system in vivo with [11C]DASB PET scans in GTP-cyclohydrolase deficient dopa-responsive dystonia patients. Sci Rep 12, 6292 (2022). https://doi.org/10.1038/s41598-022-10067-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10067-5
This article is cited by
-
Cognitive and Neuropsychiatric Impairment in Dystonia
Current Neurology and Neuroscience Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.