Abstract
To overcome the limitation of short-term efficacy of virtual reality (VR), an enhanced reality (ER) analgesia, (combination of the VR, real-time motion capture, mirror therapy [MT]) involving a high degree of patients’ presence or embodiment was explored. Patients, who underwent unilateral total knee arthroplasty (TKA), received ER analgesia. The duration was 5 times a week, for 2 weeks for one group and 5 times a week, for 1 week in the other. Visual Analogue Scale (VAS) at rest and during movement, active knee range of motion (ROM) for flexion and extension were measured repeatedly. After screening 157 patients, 60 were included. Pre-interventional evaluation was performed at 6.7 days and ER was initiated at 12.4 days after surgery. Evaluation was performed at 5, 12, 33 days after the initiation of ER. Analgesia in the 2 week therapy group was effective until the third evaluation (p = 0.000), whereas in the other group, it was effective only until the second evaluation (p = 0.010). Improvement in ROM in the 2 week group was also maintained until the third evaluation (p = 0.037, p = 0.009). It could lay the foundations for the development of safe and long-lasting analgesic tools.
Similar content being viewed by others
Introduction
Analgesics are known to cause multiple adverse effects; hence, there has been a continuous research to find alternatives. However, no ideal alternative has been found yet. With the rapid development of information technology, virtual reality (VR) is emerging as an economical, safe, and convenient alternative. Its analgesic effects have been evaluated in several medical situations like burns, invasive procedures, and amputated limbs1,2,3,4. However, all previous publications were case reports, case series, or pilot trials, except a single on-going randomized control trial by Small et al.5. Moreover, these investigations evaluated only the short-term analgesic effect and there are no reports about the long-term effects. Since VR distraction analgesia is known to work at the level of the brain6, its duration is too short to be effective for long-term analgesia. If this limitation can be overcome, VR-mediated analgesia could have a wider use.
Ramachandran et al. first introduced analgesia using mirror therapy (MT) for phantom pain in 19967,8. The proposed mechanism was that MT confounded the brain through mismatched sensory inputs from the amputated arm, inducing the brain to choose visual information instead of the amputated body sensation9. MT has been used for phantom pain10, spinal cord injury induced central pain11, complex regional pain syndrome12,13, and motor weakness after stroke14,15. In 2015, it was accepted that MT could induce changes in the cortical activity of the brain16. However, its limitation was that it was difficult to provide a perfectly synchronized mirrored image, not distorted enough to induce brain plasticity17,18. It is likely that VR can overcome this limitation.
Since both, VR and MT produce brain plasticity, but through different pathways, it could be postulated that a combination of VR and MT could induce synergistic or additive analgesia. A pilot trial reported the combined use of VR and analogous MT induced analgesia, in patients with rheumatic wrist arthritis19. The authors presumed that enhanced reality (ER: combination of VR and analogous MT using real-time image processing technique) could induce stronger brain plasticity resulting in longer-lasting effects.
Methods
This prospective, single-blind (i.e. assessor-blind), parallel group, randomized (allocation ratio 1:1), single cohort clinical trial was conducted at Ulsan University Hospital from November 2013 to June 2016, registered at FDA clinical trial registry (NCT01979718 released on November 3rd 2013), and it was approved and confirmed that all researches were performed in accordance with relevant guidelines and regulations by Ulsan university hospital institutional review board (UUH 2013-06-614). Patients who underwent unilateral total knee arthroplalsty (TKA) were included after providing informed consents for study participation and for inclusion of identifying images in an online open-access publication. Patients with a limited movement of the non-operated leg due to musculo-skeletal or neurological diseases, those who could not look at a monitor due to visual disturbance, could not understand visual analogue scale (VAS), and refused participation, were excluded. The recruited patients were randomly allocated to full term intervention group (FTI: intervention was provided shortly after physiotherapy for 5 weekdays over 2 weeks) or half term intervention group (HFI: intervention was provided for 1 week) by sequentially numbered containers, which were delivered to a physiotherapist in a sealed envelope, shortly before initiating the intervention. The numbers were generated using Wichmann-Hill random number algorithm. In addition to the intervention, all the patients in both groups received identical physiotherapy composing infrared radiation (wavelength 770 to 1500 nm, distance 45 cm from the patient’s body, perpendicular to the body surface) and continuous passive range of motion (ROM) exercises for 20 minutes, over 2 weeks. The patients were also encouraged to take a walk with or without an assistive device. Data of age, sex, weight, height, days from the pre-operative evaluation to surgery, educational status, Short Form Geriatric Depression Scale (SFGDS)20, VAS at rest and during movement, active range of movement of flexion and extension21, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)22, graded ambulation distances23, 6 minute walk test24, and timed-stands test25, were collected by another physiatrist, who was blind to the allocation. Scores of all above scales, except SFGDS, and total weekly amounts of intravenous tramadol demands, adverse effects (dizziness, psychiatric distress, boredom, depressive mood, muscles twitching26,27, and nausea28) were recorded shortly after completion of each one-week session and 3 weeks after completion of the final intervention by the same physiatrist.
Standardization of surgery and analgesia
The TKA was performed using the posterior-stabilized cruciate sacrificing method, with a medial para-patellar approach and patellar distraction. The implants were of fixed bearing type, Nexgen LPS (Zimmer, Warsaw,IN, USA), and were cemented with Simplex P (Howmedica, Rutherford, NJ), which covered the entire inner surface of the femoral implant, aligned with femoral and tibial alignment guides29. Standardized general anesthesia with no long-acting opioids was induced by injection of rocuronium (0.6–0.8 mg/kg), propofol (2–2.5 mg/kg), and alfentanil (15 μg/kg), maintained with 1–2% sevoflurane and 40–60% oxygen-nitrous oxide, and reversed with glycopyrrolate (7 μg/kg) and neostigmine (40 μg/kg). Alfentanil (0.25 mg) was infused intravenously if the heart rate or mean arterial pressure was more than 125% of pre-operative values. 10 mL of 1% lidocaine with epinephrine was infiltrated at the incision area for the surgical portals before the start of surgery30.
Based on earlier reports by Ritchie et al.31 and Hwang et al.30, analgesia was provided using celecoxib (200 mg), twice daily; acetaminophen (325 mg) and tramadol hydrochloride (37.5 mg), thrice daily; tramadol hydrocholoride (50 mg) was intravenously provided until the VAS was 40 or below. The standardized analgesia was also maintained until the patients’ first visit to the out-patient clinics.
Enhanced reality
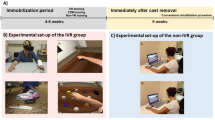
The patients were seated in a chair, thighs and trunk secured with Velcro straps, and both hands were placed on a table with spine erect, hips and knees flexed to 90 degree, and soles at 10 cm above the floor. A camera was located below the table and in front of the patient, and the distance from the patients was adjusted to allow reconstruction of virtual limbs identical to both legs, thighs, and their origin from the pelvis (Fig. 1). The intervention consisted of two sequences. The first was acquisition of a real-time embodiment and the second was of virtual limbs presence. The patients were requested to repeat voluntary free ROM movement of knee flexion and extension for 5 minutes without limitation of counts, looking at a screen showing the real-time images of their legs. Validation of embodiment of the real-time image was evaluated in random sequences32 (Appendix). After 5 minute breaks, the same session was repeated, but was limited to only 50 repetitions of flexion and extension, looking at the virtual limbs (Fig. 2). Validation of the virtual limbs presence was checked in random sequences again. A detailed description about ER can be found in Appendix.
A schematic view of the enhanced reality setup. It consists of a patient positioning tool, a screening tool, an image acquisition unit, an image processing unit, and an image displaying unit. The patient positioning tool and screening tool used a conventional table and chair. A conventional WebCam, a conventional computer, a lab-made image processing program, and a conventional monitor functioned as the image acquisition tool, image processing unit, and image displaying unit.
The flipped image by the processing unit of enhanced reality. The processing unit flips half of the acquired image, and copies it to the half of the acquired image. The left figure and center figure show a tested image before and after flipping. The right figure shows a patient’s flipped leg movement image during one’s intervention.
Statistical analyses
Based on an earlier report by Perera et al.33, authors defined a distance of 50 meters in 6 minute walk test, as the minimum mean difference of significance, and calculated standardized difference (0.71) using standard deviation (70) based on an earlier report by Boardman et al.34. Authors assumed power at 80%, α 95%, and calculated that the number of recruited patients required is 25 per group using Altman’s nomogram35, added five more patients (20% of likely dropout ratio), and finally planned to assign 30 patients to each group. Statistical Package for the Social Sciences 21.0 KO for Windows was used for the bivariate analyses. Two-sample student’s t-test, after transformation into the log in case of no normal distribution, was used to analyze the continuous variables. Wilcoxon-Mann-Whitney U test was used for the ordinal variables, repeated measures analysis of variance (ANOVA) and post hoc analysis was used to analyze the differences in time within the group and between groups.
Results
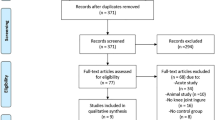
One hundred fifty seven patients who underwent unilateral TKA were assessed for eligibility, and 97 were excluded. Thirty patients were allocated to each group. Four patients were dropped during the first session (demand for analgesics: 3, toxic hepatitis: 1), nine during the second session (toxic hepatitis: 2, early discharge: 7), and five during the third session (no visit in out-patient clinic). Twenty-two in the FTI group and 20 in the HTI group were analyzed finally (Fig. 3). No significant difference in demographic characteristics was found (Table 1).
Validation of real-time embodiment and virtual limb presence
In the first session, real-time embodiment of the image scored 4.0, virtual reality presence scored 4.0, and the sham stimulation −4.5. In the second session, the real-time embodiment was 4.1, virtual reality presence 3.9, the sham stimulation −4.2 (Table 2).
Functional scales and painkiller usage
No significant difference was found between the pre-interventional evaluation and the evaluation at the three time points after intervention (Table 3). The routine provision with the standardized analgesia controlled the patients’ pain so enough during the second weeks of the interventions that no intravenous injections of tramadol hydrocholoride happened. Change in WOMAC scale showed no significant differences in time within the group and between groups (p = 0.081, F: 11.53 and p = 0.078, F: 9.61). All the patients showed 4 grades in the graded ambulation distance.
Difference in duration of analgesia and ROM
The FTI group showed a continuous and significant change in VAS at rest over 5 weeks (p = 0.000, F: 23.65). However, the HTI group showed significant change in VAS only between the first and second week (p = 0.010, F: 6.90) (Fig. 4a). The VAS on movement showed similar results: significant improvement in the FTI group over 5 weeks (p = 0.000, F: 28.39) and in the HTI group improvement was seen only between the first and second week (p = 0.008, F: 7.43) (Fig. 4b). No significance was found in VAS analyses between the groups.
Difference in duration of analgesia and ROM. (a) and (b) Continuous significant improvement of the VAS at rest and movement was seen over 5 weeks in the FTI group and improvement in the HTI was limited to 2 weeks. (c) Only the FTI group showed continuous significant improvement of active knee flexion over 5 weeks. (d) Continuous significant improvement of active knee extension was seen over 5 weeks in the FTI and improvement in the HTI was limited to 2 weeks. FTI: full term intervention group, HTI; half term intervention group. *p < 0.05, **p < 0.01.
A significant difference in time within the group was demonstrated in active ROM of knee flexion and extension in the FTI group (p = 0.037, F = 4.91 and p = 0.009, F = 7.18), but group-by-time interaction in ROMs was not noticed. The post hoc analysis revealed that a continuous and significant improvement in active ROM of flexion and extension was found over 5 weeks in the FTI group, but significant improvement in active ROM of extension was found only between the first and second week in the HTI group (Fig. 4d).
Discussion
Improvement in VAS at rest or during movement was noticed in both groups. This is similar to previous reports of VR1,2,3,4. However all previous reports were case-series or crossover trials, and evaluated only the immediate analgesia during ROM exercises, burn wound debridement, or chemotherapy. Two systemic reviews concluded that VR has the potential to be an useful analgesic tool, but more evidence is required36,37. With no randomized controlled trials except a single on-going trial5, it is important to check the long-lasting effect. In comparison within the group, the FTI group showed a significant improvement which was maintained at 3 weeks after discontinuation of the intervention; however, the improvement in HTI group lasted only one week. Considering the duration of the intervention in the two groups (HTI: one week vs. FTI: two weeks), this indicates that long-lasting analgesia is dose-dependent. A few trials evaluated the effectiveness of analgesia with multiple VR sessions38,39. Hoffman et al. reported there was no decrease in pain until 7 sessions, in 7 burn patients performing ROM exercises38. Faber et al. found that analgesia was maintained significantly until the third day, but not beyond, in 36 patients undergoing burn dressing39. However, their evaluation period was too short following the VR to be comparable with the current results.
The current long-lasting effect can be explained through the pathway of induction of brain plasticity in brief. The VR induces analgesia through distraction phenomena acting on the pain matrix (the anterior cingulate cortex, primary and secondary somatosensory cortex, insula, thalamus)6,40,41. It is known that neuroplasticity can be induced in response to stimuli in a matured adult brain42,43 and pain can be one of these stimuli44,45. Similarly the MT induces change in cortical activity of the human brain16, and it has been used in various medical fields10,11,12,13,14,15. Visual input dominates other somatosensory efferent signals for proprioceptive perception of the brain46. MT confounds the brain32, which recognized the reflected visual feedback as a well-functioning limb image, and induces neuro-plasticity of the brain in charge of the contralateral body47,48,49. Considering that the motor cortex activation provides analgesia50 and that the motor cortex gets activated even by looking at the movement of another person’s extremity51, in the current trial it might have induced synergistic analgesia with VR distraction, by watching the image of a well-functioning operated leg. Similar to the current trial, VR using the concept of MT has been attempted earlier. Desmond et al. reported that immediate analgesia was noticed in 1 of 3 patients due to VR, in which the virtual forearm controlled by the contralateral hand, was superimposed on the amputated arm50. Cole et al. reported analgesia in 10 amputations among 14 leg or arm amputations using a similar design52. Only one trial, reported long-lasting analgesia of 1 week between sessions, in a protocol of 5 sessions, once a week. The VR mirror visual feedback induces analgesia lasted for 1 week in 4 out of 5 patients with complex regional pain syndrome53. However, imaginary movements alone could exacerbate pain in spinal cord injury54 or complex regional pain syndrome55. The above trials used augmented virtual limbs, rather than real limbs like the MT; however, this could lead to poor embodiment. Considering that high degree of embodiment in the MT56,57 and the sense of presence in the VR distraction37,53,58 are essential, it is critical that the stimuli should be strong enough to evoke the brain plasticity52 and the validity of embodiment and the sense of presence might be the determining factors for better results.
Most trials on VR distraction analgesia used a single self-reporting question for validity of the presence. Six patients reported the strongest feeling of going-inside the VR among 11 patients undergoing the VR during burn wound debridement in a trial by Hoffman et al.2 and 8 felt virtual sensation among 14 amputated patients53. The mean value of validity of presence was 56 (0 to 100 rating) during the VR in 7 burn patients who took ROM exercises38. Moreover, all earlier reports were preliminary. Although a questionnaire with multiples parameters used by Witmer et al. showed higher reliability59, it was used only in a single clinical trial of 8 pediatric burn patients in which the mean score was 4.8 (0 to 7 rating)60. However, authors used a questionnaires consisting of six items, to check for real-time embodiment, virtual limb presence, and sham stimuli (−5 [not at all] to 5 [perfect] rating)32. In the first session, the real-time embodiment scored 4.0, the virtual limb 4.0, and the sham stimulation −4.5, and in the second session, the real-time embodiment was 4.1, the virtual limb 3.9, the sham stimulation −4.2. These scores were much higher than scores of the previous trials, indicating that ER might induce long-lasting analgesia through more potentiated brain plasticity.
Comparing the ROM improvement within the group, similar results were noticed: The FTI group showed significant improvement until 3 weeks after the discontinuation of the intervention, whereas the improvement in the HTI group was only for one week after. Sarig-Bahat et al. reported that a single session of VR, in which each patient was encouraged to catch up flies with spray, increased cervical ROM (generally 10 degree or more) in 67 patients with/without neck pain61. This is in accordance with the current results, however, only the immediate effect was evaluated. Considering that the same VR showed an error range in ROM assessment of up to 7.2° in flexion and extension and to 16.1° in rotation62, the current trial may be the first evidence that the VR improves active ROM.
No major or minor adverse event was noticed although simulator sickness in the VR28,38 and dizziness, emotional discomfort, depressive mood, and muscle twitching in the MT26,27 were reported. However, objective evaluation, similar to a study by Keshavarz and Hecht63, was not performed.
The current study had several limitations. Per-protocol analysis was used instead of intention-to-treat analysis, because the protocol was violated in 18 patients. The effect size could be overvalued by per-protocol analysis64 and the large number of dropouts (18 of 60), which is a high number for a study of this size. Majority (76%) of the patients in the current trial were females. Considering the difference in visual perception between sexes65,66,67, no sub-group analyses regarding sex may be warranted. Although a significant difference in time within the group over 5 weeks in VAS and active ROM of the knee was approved in the current trial, it could not gain generalizability because its action induced no significant improvement of the functional scales and was restricted to the knee, which the intervention was targeted towards. It could be because the period of intervention was not long enough; hence, a clinical trial is necessary to determine the therapeutic time window and dosage. Another limitation was, not checking the degree of neuroplasticity using electro-diagnostic or imaging evaluation.
References
Hoffman, H. G. et al. Virtual reality pain control during physical therapy range of motion exercises for a patient with multiple blunt force trauma injuries. Cyberpsychology & behavior: the impact of the Internet, multimedia and virtual reality on behavior and society 12, 47–49, https://doi.org/10.1089/cpb.2008.0056 (2009).
Hoffman, H. G. et al. Virtual reality pain control during burn wound debridement in the hydrotank. The Clinical journal of pain 24, 299–304, https://doi.org/10.1097/AJP.0b013e318164d2cc (2008).
Schneider, S. M., Kisby, C. K. & Flint, E. P. Effect of virtual reality on time perception in patients receiving chemotherapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer 19, 555–564, https://doi.org/10.1007/s00520-010-0852-7 (2011).
Maani, C. V. et al. Virtual reality pain control during burn wound debridement of combat-related burn injuries using robot-like arm mounted VR goggles. The Journal of trauma 71, S125–130, https://doi.org/10.1097/TA.0b013e31822192e2 (2011).
Small, C., Stone, R., Pilsbury, J., Bowden, M. & Bion, J. Virtual restorative environment therapy as an adjunct to pain control during burn dressing changes: study protocol for a randomised controlled trial. Trials 16, 329, https://doi.org/10.1186/s13063-015-0878-8 (2015).
Li, A., Montano, Z., Chen, V. J. & Gold, J. I. Virtual reality and pain management: current trends and future directions. Pain management 1, 147–157, https://doi.org/10.2217/pmt.10.15 (2011).
Ramachandran, V. S. & Rogers-Ramachandran, D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 263, 377–386, https://doi.org/10.1098/rspb.1996.0058 (1996).
Ramachandran, V. S., Rogers-Ramachandran, D. & Cobb, S. Touching the phantom limb. Nature 377, 489–490, https://doi.org/10.1038/377489a0 (1995).
Ramachandran, V. S. & Altschuler, E. L. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 132, 1693–1710, https://doi.org/10.1093/brain/awp135 (2009).
Chan, B. L. et al. Mirror therapy for phantom limb pain. N Engl J Med 357, 2206–2207, doi:357/21/2206 (2007).
Moseley, G. L. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain 130, 294–298, doi:S0304-3959(07)00014-0 (2007).
Karmarkar, A. & Lieberman, I. Mirror box therapy for complex regional pain syndrome. Anaesthesia 61, 412–413, doi:ANA4605 (2006).
McCabe, C. S. et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford) 42, 97–101 (2003).
Altschuler, E. L. et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036, doi:S0140673699009204 (1999).
Yavuzer, G. et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil 89, 393–398, https://doi.org/10.1016/j.apmr.2007.08.162 (2008).
Lee, H. M., Li, P. C. & Fan, S. C. Delayed mirror visual feedback presented using a novel mirror therapy system enhances cortical activation in healthy adults. Journal of neuroengineering and rehabilitation 12, 56, https://doi.org/10.1186/s12984-015-0053-1 (2015).
Brodie, E. E., Whyte, A. & Niven, C. A. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. European journal of pain 11, 428–436, https://doi.org/10.1016/j.ejpain.2006.06.002 (2007).
Hunter, J. P., Katz, J. & Davis, K. D. The effect of tactile and visual sensory inputs on phantom limb awareness. Brain 126, 579–589 (2003).
Choi, S. W. M. D., Ph.D, Heo, S., Hwang, C. H. M. D., Ph.D & Koo, K.-in. Ph.D. Mirror Therapy Using Virtual Reality on the Wrsit of Rheumatoid Arthritis; Pilot Trial. Brain & NeuroRehabilitation 9, 48–55 (2016).
Greenberg, S. A. How to try this: the Geriatric Depression Scale: Short Form. Am J Nurs 107, 60–69; quiz 69–70, https://doi.org/10.1097/01.NAJ.0000292204.52313.f3 (2007).
Norkin C, W. J. Measurement of joint motion: a guide to goniometry., (F. A. Davis Company, 1995).
Bellamy, N., Buchanan, W. W., Goldsmith, C. H., Campbell, J. & Stitt, L. W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15, 1833–1840 (1988).
Good, R. P., Snedden, M. H., Schieber, F. C. & Polachek, A. Effects of a preoperative femoral nerve block on pain management and rehabilitation after total knee arthroplasty. Am J Orthop 36, 554–557 (2007).
Harada, N. D., Chiu, V. & Stewart, A. L. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil 80, 837–841, doi:S0003-9993(99)90236-8 (1999).
Newcomer, K. L., Krug, H. E. & Mahowald, M. L. Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol 20, 21–27 (1993).
Darnall, B. D. & Li, H. Home-based self-delivered mirror therapy for phantom pain: a pilot study. J Rehabil Med 44, 254–260, https://doi.org/10.2340/16501977-0933 (2012).
Casale, R., Damiani, C. & Rosati, V. Mirror therapy in the rehabilitation of lower-limb amputation: are there any contraindications? Am J Phys Med Rehabil 88, 837–842, https://doi.org/10.1097/PHM.0b013e3181b74698 (2009).
Sharar, S. R. et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from 3 ongoing studies. Arch Phys Med Rehabil 88, S43–49, https://doi.org/10.1016/j.apmr.2007.09.004 (2007).
Cho, S. D. & Hwang, C. H. Improved single-limb balance after total knee arthroplasty. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 21, 2744–2750, https://doi.org/10.1007/s00167-012-2144-x (2013).
Youm, Y. S., Cho, S. D. & Hwang, C. H. Prospective, double-blind, randomized controlled trial of electrophysiologically guided femoral nerve block in total knee arthroplasty. Ther Clin Risk Manag 9, 107–113, https://doi.org/10.2147/TCRM.S33544 (2013).
Ritchie, E. D. et al. Suprascapular nerve block for postoperative pain relief in arthroscopic shoulder surgery: a new modality? Anesth Analg 84, 1306–1312 (1997).
Romano, D., Bottini, G. & Maravita, A. Perceptual effects of the mirror box training in normal subjects. Restor Neurol Neurosci, doi:A4LW5504H1324153 (2013).
Perera, S., Mody, S. H., Woodman, R. C. & Studenski, S. A. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54, 743–749, doi:JGS701 (2006).
Boardman, D. L., Dorey, F., Thomas, B. J. & Lieberman, J. R. The accuracy of assessing total hip arthroplasty outcomes: a prospective correlation study of walking ability and 2 validated measurement devices. J Arthroplasty 15, 200–204, doi:S0883-5403(00)90242-0 (2000).
DG, A. Practical statistics for medical research. (Chapman and Hall, 1991).
Morris, L. D., Louw, Q. A. & Grimmer-Somers, K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. The Clinical journal of pain 25, 815–826, https://doi.org/10.1097/AJP.0b013e3181aaa909 (2009).
Malloy, K. M. & Milling, L. S. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clinical psychology review 30, 1011–1018, https://doi.org/10.1016/j.cpr.2010.07.001 (2010).
Hoffman, H. G., Patterson, D. R., Carrougher, G. J. & Sharar, S. R. Effectiveness of virtual reality-based pain control with multiple treatments. The Clinical journal of pain 17, 229–235 (2001).
Faber, A. W., Patterson, D. R. & Bremer, M. Repeated use of immersive virtual reality therapy to control pain during wound dressing changes in pediatric and adult burn patients. Journal of burn care & research: official publication of the American Burn Association 34, 563–568, https://doi.org/10.1097/BCR.0b013e3182777904 (2013).
Hoffman, H. G. et al. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport 15, 1245–1248 (2004).
Hoffman, H. G. et al. Using FMRI to study the neural correlates of virtual reality analgesia. CNS spectrums 11, 45–51 (2006).
Draganski, B. et al. Neuroplasticity: changes in grey matter induced by training. Nature 427, 311–312, https://doi.org/10.1038/427311a (2004).
May, A. et al. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex 17, 205–210, https://doi.org/10.1093/cercor/bhj138 (2007).
May, A. Chronic pain may change the structure of the brain. Pain 137, 7–15, https://doi.org/10.1016/j.pain.2008.02.034 (2008).
Woolf, C. J. & Salter, M. W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000).
Graziano, M. S. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proceedings of the National Academy of Sciences of the United States of America 96, 10418–10421 (1999).
Franceschini, M. et al. Mirror neurons: action observation treatment as a tool in stroke rehabilitation. Eur J Phys Rehabil Med 46, 517–523, doi:R33102276 (2010).
Garrison, K. A., Winstein, C. J. & Aziz-Zadeh, L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair 24, 404–412, https://doi.org/10.1177/1545968309354536 (2010).
Small, S. L., Buccino, G. & Solodkin, A. The mirror neuron system and treatment of stroke. Dev Psychobiol 54, 293–310, https://doi.org/10.1002/dev.20504 (2012).
Pleger, B. et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett 356, 87–90 (2004).
Ehrsson, H. H., Geyer, S. & Naito, E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. Journal of neurophysiology 90, 3304–3316, https://doi.org/10.1152/jn.01113.2002 (2003).
Sato, K. et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain medicine 11, 622–629, https://doi.org/10.1111/j.1526-4637.2010.00819.x (2010).
Cole, J., Crowle, S., Austwick, G. & Slater, D. H. Exploratory findings with virtual reality for phantom limb pain; from stump motion to agency and analgesia. Disability and rehabilitation 31, 846–854, https://doi.org/10.1080/09638280802355197 (2009).
Gustin, S. M. et al. Movement imagery increases pain in people with neuropathic pain following complete thoracic spinal cord injury. Pain 137, 237–244, https://doi.org/10.1016/j.pain.2007.08.032 (2008).
Moseley, G. L. Imagined movements cause pain and swelling in a patient with complex regional pain syndrome. Neurology 62, 1644 (2004).
Rohde, M., Di Luca, M. & Ernst, M. O. The Rubber Hand Illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One 6, e21659, https://doi.org/10.1371/journal.pone.0021659 (2011).
Moseley, G. L., Gallace, A. & Spence, C. Is mirror therapy all it is cracked up to be? Current evidence and future directions. Pain 138, 7–10, https://doi.org/10.1016/j.pain.2008.06.026 (2008).
Hoffman, H. G. et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain 111, 162–168, https://doi.org/10.1016/j.pain.2004.06.013 (2004).
Singer, B. G. W. A. M. J. Measuring Presenece in Virtual Environments; A Presence Questionnare. Presence 7, 225–240 (1998).
Chan, E. A., Chung, J. W., Wong, T. K., Lien, A. S. & Yang, J. Y. Application of a virtual reality prototype for pain relief of pediatric burn in Taiwan. Journal of clinical nursing 16, 786–793, https://doi.org/10.1111/j.1365-2702.2006.01719.x (2007).
Sarig-Bahat, H., Weiss, P. L. & Laufer, Y. Neck pain assessment in a virtual environment. Spine 35, E105–112, https://doi.org/10.1097/BRS.0b013e3181b79358 (2010).
Sarig-Bahat, H., Weiss, P. L. & Laufer, Y. Cervical motion assessment using virtual reality. Spine 34, 1018–1024, https://doi.org/10.1097/BRS.0b013e31819b3254 (2009).
Keshavarz, B. & Hecht, H. Validating an efficient method to quantify motion sickness. Human factors 53, 415–426 (2011).
Melander, H., Ahlqvist-Rastad, J., Meijer, G. & Beermann, B. Evidence b(i)ased medicine–selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications. Bmj 326, 1171–1173, https://doi.org/10.1136/bmj.326.7400.1171 (2003).
Cadieux, M. L., Barnett-Cowan, M. & Shore, D. I. Crossing the hands is more confusing for females than males. Exp Brain Res 204, 431–446, https://doi.org/10.1007/s00221-010-2268-5 (2010).
Barnett-Cowan, M., Dyde, R. T., Thompson, C. & Harris, L. R. Multisensory determinants of orientation perception: task-specific sex differences. Eur J Neurosci 31, 1899–1907, https://doi.org/10.1111/j.1460-9568.2010.07199.x (2010).
Linn, M. C. & Petersen, A. C. Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev 56, 1479–1498 (1985).
Acknowledgements
Authors give our thanks to Eun Ji Park, M.Sc., who worked in Ulsan University Hospital, for her help to analyze the data, and also to Sun cheol Heo, who studied at Department of Biomedical Engineering, University of Ulsan, for his help to develop the image processing program, and Lee Ju Youn, physiotherapist, who worked in Ulsan University Hospital, for conducting ER. This paper is the result of research project of Basic Science Research Program, through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future, Planning (NRF-2017R1D1A1B03034982) and of the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (NRF-2017R1A2B4011478).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the paper. K.I.K.: 1 (conception and design), 2 (revising), 3 (final approval), 4 (integrity) D.K.P.: 1 (design), 2 (drafting the work), 3 (final approval), 4 (accuracy) Y.S.Y.: 1 (conception), 2 (drafting the work), 3 (final approval), 4 (integrity) S.D.C.: 1 (conception), 2 (drafting the work), 3 (final approval), 4 (integrity) C.H.H.: 1 (conception and design), 2 (revising), 3 (final approval), 4 (integrity).
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koo, Ki., Park, D.K., Youm, Y.S. et al. Enhanced Reality Showing Long-Lasting Analgesia after Total Knee Arthroplasty: Prospective, Randomized Clinical Trial. Sci Rep 8, 2343 (2018). https://doi.org/10.1038/s41598-018-20260-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20260-0
Keywords
This article is cited by
-
Effectiveness of Virtual Reality–Based Rehabilitation Interventions in Improving Postoperative Outcomes for Orthopedic Surgery Patients
Current Pain and Headache Reports (2024)
-
Effectiveness of motor imagery for improving functional performance after total knee arthroplasty: a systematic review with meta-analysis
Journal of Orthopaedic Surgery and Research (2022)
-
Post-operative rehabilitation using a digital healthcare system in patients who had undergone rotator cuff repair: protocol for a single-center randomized controlled trial
Trials (2022)
-
The influence of perioperative interventions targeting psychological distress on clinical outcome after total knee arthroplasty
Rheumatology International (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.