Abstract
Purpose
To report the clinical features and treatment outcomes in a cluster of patients with endophthalmitis after cataract surgery caused by nontuberculous mycobacterium.
Patients and methods
Retrospective chart review and noncomparative, consecutive case series. Nine consecutive cases of endophthalmitis, after cataract surgery in a local clinic, were referred to our hospital. The treatment outcomes and analysis of risk factors for infection are reported.
Results
The major symptoms at presentation were pain, redness, and decreased vision. Best-corrected visual acuity at presentation ranged from hand motion in two cases (22%), counting fingers at 30 cm in three cases (33%), 20/100 in two cases (20%), 20/63 in one case (11%), to 20/50 in one (11%) case. The mean duration between cataract surgery to presentation at our hospital was 16.7 days. Prompt intravitreal injections (IVI) of amikacin (0.4 mg/0.1 mL) and vancomycin (1 mg/0.1 mL), with topical moxifloxacin were administered initially. Pars plana vitrectomy with amikacin (10 mg/L) and vancomycin (20 mg/L) intravitreal irrigation, and intraocular lens removal were performed for all patients. Systemic antibiotics including amikacin and tigecycline were prescribed for 10 days, and clarithromycin was prescribed for at least 3 months. In all the nine cases, the culture results from either aqueous tapping or vitrectomy sample were positive for nontuberculous Mycobacterium: Mycobacterium abscessus/chelonae, which was compatible with iatrogenic clustered infection. At the last follow-up, three cases (33.3%) had best-corrected visual acuity of counting fingers at 30 cm, while the other six cases had no light perception. Two cases (22%) were enucleated and one case (11%) had phthisis bulbi.
Conclusion
Nontuberculous mycobacterium endophthalmitis (NTME) often induces chronic recurrent or persistent intraocular inflammation. Very poor outcomes despite aggressive antibiotic treatment and repeated surgical interventions are suggestive of the virulent nature of the organisms. Autoclave sterilization and perioperative disinfection may help in reducing iatrogenic clustered infection.
Similar content being viewed by others
Introduction
Postoperative endophthalmitis remains a serious complication after cataract surgery with an overall incidence of 0.14% [1]. The causative microorganism of postoperative endophthalmitis is often the most important factor influencing the visual outcomes of the patients.
Nontuberculous mycobacteria (NTM), or atypical mycobacteria, are aerobic, nonmotile, non-spore forming, rapidly growing, and acid fast bacilli that are widely distributed in the natural and hospital environment [2]. It can cause many ocular infections, such as infectious keratitis, conjunctivitis, scleritis, uveitis, and endophthalmitis [3, 4].
NTM is considered a rare cause of endophthalmitis, with only 68 cases reported before 2016. In this paper, we report the clinical features and treatment outcomes of a cluster of nontuberculous mycobacterium endophthalmitis cases after cataract surgery.
Methods
This study was approved by the institutional review board of Tri-Service General Hospital. Informed consent was obtained from all patients. It is a retrospective, consecutive, noncomparative review of medical and microbiology records of all patients diagnosed and treated for NTM endophthalmitis.
Nine consecutive patients underwent cataract surgery (phacoemulsification with intraocular lens implantation) in one local ophthalmic clinic in Taipei, Taiwan from 12 to 22 September 2014. The patients had been prescribed topical moxifloxacin and prednisolone acetate eye drops every 2 h after surgery for at least 1 week. When they were suspected to have endophthalmitis after cataract surgery, they were transferred to our hospital. Patient data, including age at presentation, sex, history of immunosuppression, cause and duration of symptoms, Snellen best-corrected visual acuity at presentation and last visit, clinical features, microbiology findings, medical and surgical management, and visual outcomes, are listed in Table 1. All patients underwent slit lamp biomicroscopy, indirect ophthalmoscopy, and B-scan ultrasonography.
Results
A total of nine culture-positive NTM cases were analyzed. The mean age at presentation was 69 years (range, 59–82 years). There was a female predilection with a male-to-female ratio of 2:7. The mean follow-up duration was 10.9 months (range, 3–34 months). Mean duration of incubation time was 16.7 days (range, 8–20 days). All nine patients have not received any intraocular interventions other than cataract surgery. A history of systemic illness was present in four of nine patients (44.4%): three (3/9; 33%) with hypertension (HTN) only and one (1/9; 11%) with HTN and type 2 diabetes mellitus (DM). One patient (Case 4) had chronic hepatitis B, another (Case 3) had senile dementia, and another (Case 1) had bilateral chronic angle-closure glaucoma.
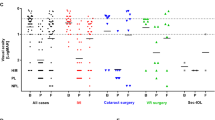
For all patients, the major symptoms at presentation were pain, redness, and decreased vision. Visual acuity at presentation ranged from hand motion in two patients (22%), counting fingers at 30 cm in three patients (33%), 20/200 in two patients (20%), 20/63 in one patient (11%), and 20/50 in one patient (11%). Cornea edema, keratic precipitate (KP), marked anterior chamber reaction, hypopyon, cyclitic membrane with fibrotic exudates and white plaques covering the intraocular lens (IOL), and severe vitreous humor inflammatory reaction were observed in almost all patients (Table 1 and Figs. 1a, b, 2, and 3). Initial B-scan ultrasound results showed moderate reflective membranous echoes in the vitreous fluid with attached retina in all patients.
(a, left) A 75-year-old woman (Case 3) presented with marked cyclitic membrane over the pupil margin, hypopyon, and white plaques of various sizes within the capsular bag. (b, right) After two pars plana vitrectomy with removal of the intraocular lens, intravitreal antibiotics irrigation, and four intravitreal injections of amikacin, the anterior segment inflammatory reaction and hypopyon subsided. The aqueous culture result was positive initially and negative at final intervention
Microbiology evaluation
All nine cases had a positive culture of Mycobacterium chelonae/Mycobacterium abscessus from intraocular fluid (either aqueous humor or vitreous fluid or both). The antibiotic susceptibility testing was performed with a minimum inhibitory concentration (MIC) of 8 μg/mL for amikacin, which was much lower than the actual dose (80 μg/mL) we used for IVI.
Medical and surgical treatment
In all the cases, prompt IVI of amikacin (0.5 mg/0.1 mL) and vancomycin (1 mg/0.1 mL) were administered at first visit when endophthalmitis was highly suspected after anterior chamber and vitreous samples were collected for culture. Pars plana vitrectomy (PPV) was performed in all cases. We performed antibiotic irrigation (amikacin 10 mg/L and vancomycin 20 mg/L) and intravitreal injection (amikacin 0.5 mg/0.1 mL and vancomycin 1 mg/0.1 mL). One patient (Case 9) was administered intravitreal betamethasone (0.4 mg/0.1 mL) injection along with antibiotics during the second intervention. IOL removal was performed for all nine cases at either first or second vitrectomy. All patients were then started on topical moxifloxacin, tobramycin, and prednisolone acetate eye drops and systemic antibiotics (oral clarithromycin 500 mg twice a day, intravenous tigecycline 50 mg twice a day, and amikacin 750 mg per day) for various treatment courses. During the course of treatment, all nine patients underwent one or more pars plana vitrectomy with intravitreal and anterior chamber irrigation (amikacin 10 mg/L and vancomycin 20 mg/L). Additional surgeries for pars plana vitrectomy with antibiotic irrigation were performed, depending on the clinical status and culture results. IVIs of amikacin were administered with an average of 9.9 injections per patient (range, 6–22 injections). The average treatment duration of surgical interventions (including IVI) was 3.3 months. The treatment course was extended to 7 months for Case 9.
Clinical diagnosis of nontuberculous mycobacterium infection (M. abscessus) was confirmed by positive culture of intraocular fluid sample or by standard laboratory protocol, including smear preparation and inoculation of culture media in all nine cases.
Case 9
Case 9, a 60-year-old woman, was the last case referred from the ophthalmic clinic. She presented at our hospital 17 days after cataract surgery with decreased vision, pain, and redness of the left eye. The BCVA of the left eye was 20/100. Moderate corneal edema, severe AC reaction with 1 mm hypopyon, cyclitic membrane on pupil and IOL, and cloudy vitreous with cellular infiltration were noted. Because of the experiences of the previous eight cases, NTM endophthalmitis was highly suspected initially. We gave her IVI with vancomycin 1 mg/0.1 mL + amikacin 0.4 mg/0.1 mL on the day of presentation. Topical vancomycin and prednisolone acetate and oral clarithromycin 500 mg q12h were administered empirically. However, worse symptoms with BCVA deteriorating to counting fingers, more severe AC reaction, and dense cyclitic membrane developed on the following days. The second IVI was performed with amikacin 0.4 mg/0.1 mL 1 week later. The aqueous tapping culture of the first IVI grew M. abscessus/M. chelonae. Additional two IVIs with amikacin were performed within the next week. However, persistent intraocular reaction with cornea edema was noted. PPV with explantation of IOL capsular bag and antibiotic irrigation (amikacin 10 µg/mL and vancomycin 20 µg/mL) were performed 15 days after presentation. Meanwhile, intravenous tigecycline 50 mg every 12 h and amikacin 750 mg every day were added based on the antibiotic susceptibility testing and the suggestion of an infectious disease specialist. We collected samples for culture on every IVI and PPV performed, and the results were all positive for M. abscessus.
Several days after PPV and removal of the IOL, the BCVA became 20/200, the AC had fewer inflammatory cells (+) and there was little mutton fat KP. However, greater AC reaction with grade 2+ cells and more KP developed 2 weeks later. Intravitreal injection of amikacin was administered due to concerns about the possible recurrence of endophthalmitis. However, there was still moderate AC reaction and BCVA decreased to counting fingers in the following 2 weeks. A second PPV with antibiotic irrigation was performed 1 month later. The vitreous culture result was negative. After the second PPV, the BCVA improved to 20/63; AC had fewer inflammatory cells but low-to-moderate grade flare and cells, and mutton fat KP were still noted in the following months, despite frequent IVI every week or every other week.
After 4 months, the intraocular inflammation was exacerbated, with BCVA dropping to 20/630, grade 3+ cells and hypopyon 1 mm. A third PPV with antibiotic irrigation (amikacin and vancomycin) was performed. The symptoms did not improve; vision progressively worsened to light perception and there was severe corneal edema, severe intraocular inflammation, and hypopyon in the following month. Enucleation was eventually performed.
In summary, we performed three PPV, removed the IOL and capsule, and administered 22 IVIs in the whole treatment course for Case 9. However, poor response of the eye to the administered antibiotics was noted despite aggressive treatment.
Visual and anatomic outcome
At last follow-up, three of nine patients (33.3%) had BCVA of counting fingers at 30 cm. The other six patients had no light perception. Enucleation was performed in two of nine patients (22.2%) and one patient (5.3%) had phthisis bulbi (Table 2).
Discussion
We demonstrate clustered cases of postoperative NTM endophthalmitis (NTME) from a local clinic with very poor visual outcomes despite aggressive treatment. In all cases, initial PPV, AC irrigation, intravitreal antibiotics, and systemic antibiotics seemed to be temporarily effective with improved intraocular inflammation. However, endophthalmitis recurred repeatedly in some cases. Several patients became unresponsive to additional local and systemic antibiotics. Two patients needed enucleation.
A clustered endophthalmitis outbreak may lead to blindness in patients and potential legal problems for ophthalmologists. There are many potential sources of contamination in clustered outbreaks. These include contaminated intraprocedural solutions, both extraocular (e.g., povidone iodine and saline) and intraocular (e.g., irrigating fluid, intracameral drugs including antibiotics and viscoelastic materials), inadequate sterilization of instruments, phacoemulsification machines including tubing and phacoemulsification probes, inadequate or contaminated ventilation systems providing poor air change rate in the operating environment, defective periocular sterilization procedures, and others (e.g., defective, contaminated or dirty instruments, or postoperative eye drops). However, even with thorough investigation, in ~20% of cases, there is no obvious or identifiable source [5]. Povidone iodine solution (5%) has been shown to significantly reduce conjunctival and perilimbal flora [6]. Positive culture rates from the conjunctiva of normal eyes have been reduced from 60% or more using a combined regimen of topical antibiotics and irrigation with povidone iodine solution. Akçakaya et al. [7] observed that inadequately sterilized irrigation solutions had frequently been reported as the cause of endophthalmitis outbreaks, and ophthalmologists should never assume that any commercially available solution is sterilized. We collected samples of all the above-mentioned solutions, instruments, environment samples, and eye drops from the clinic where the outbreak occurred to detect the source of contamination. However, all possible sources of contamination had been cleaned and a new autoclave system had commenced before the samples could be collected and no positive culture results were obtained. Additionally, all medication containers tested were sterile.
Compared to endophthalmitis caused other microorganisms, NTME usually has relatively poor visual outcome and even needs enucleation in refractory cases. We reviewed case reports of NTME in the past decades; these are listed in Table 3. Including our nine cases, there were a total of 29 cases. Only 4 of the 29 cases (13.8%) had a final BCVA better than 20/200. On the other hand, there were as many as 23 patients (79.3%) with very poor final BCVA of less than 20/800 or who had lost their eyes. Its rarity often leads to initial misdiagnosis and incorrect treatment, and its resistance to treatment results in poor visual outcomes [8, 9].
Although NTM has been reported to be sensitive to antibiotics including quinolones, clarithromycin, and aminoglycosides, the susceptibility of the causative organisms may vary [10,11,12,13]. There is no international standardized antibiotic susceptibility test for NTM strains. Due to rapidly increasing resistance of NTM strains to antimicrobials, a multi-regimen empirical therapy (with combined systemic and local antibiotics) was suggested for medical treatment for NTME. Surgical debridement by PPV, removal of IOL–capsule complex with concurrent antibiotic irrigation, and IVI of antibiotics were also the suggested treatment in most published literature. The above interventions were performed and were slightly effective with an initial decrease in intraocular inflammation. However, endophthalmitis recurred and several patients became unresponsive to additional treatment. Therefore, drug resistance was highly suspected, especially in Case 9. We administered four consecutive IVI and systemic and topical antibiotics to Case 9 initially but the response was poor. The intraocular inflammation did not subside until PPV and removal of the IOL. However, low-grade intraocular inflammation was still noted for several months and culture results were positive for M. abscessus for more than 1 month despite very aggressive surgical and medical treatment. There was recurrent severe endophthalmitis without any response to treatment and the patient lost her vision. Although highly suspected, the drug resistance could not be proven by our lab.
Removal of IOL–capsule complex is another important issue. It has been reported that M. abscessus can be obtained from the IOL–capsule complex even after intravitreal injection of amikacin [14]. In all our cases, we removed the IOL–capsule complex at the first or second PPV. We noticed that the intraocular inflammation was better controlled after surgery in most cases. However, there was still low-to-moderate intraocular inflammation in some cases despite aggressive treatment. It is possible that the incomplete removal of residual lens capsule due to inflammatory adhesion may have formed a biofilm as an intraocular reservoir, impeding the penetration of antibiotics [15] and resulting in the poor visual outcome in our patients. Shah et al. [9] reported complete removal of IOL–capsule complex or complete removal of IOL with capsulectomy with better prognosis than our patients.
The incubation time is another factor that influences visual outcome. The incubation time was relatively shorter (range, 8–20 days; mean, 16.7 days) in our patients with extremely poor visual outcomes. We reviewed 20 NTME cases in the literature (Table 3) [16,17,18,19,20,21] and analyzed them together with our nine cases to see the relationship between incubation time and visual outcomes. Based on final vision, there were 21 cases with final visual acuity worse than 1/200 (the poor vision group) and eight cases with final visual acuity better than 5/200 (the better vision group). We found that the mean incubation time was 4.10 weeks in the poor vision group and 6.31 weeks in the better vision group (p < 0.05). On the other hand, based on incubation time, we have 20 cases with incubation time less than 1 month (acute group) and nine cases with incubation time longer than 1 month (chronic group). We found that 80% of cases in the acute group attained a final visual acuity worse than 1/200, while only 55.6% of cases in the chronic group had a poor final visual acuity. Accordingly, we conclude that cases of acute NTME with incubation time less than 1 month would have less favorable visual outcomes compared to chronic cases.
Corticosteroids should be used with caution in NTME. Kheir et al. reviewed 174 case reports of NTM ocular infection and concluded that eyes that received steroids prior to diagnosis of the infection were almost three times more likely to have lack of initial response to medical therapy, more likely to develop a prolonged course of infection, and less likely to resolve [10]. However, topical corticosteroid usage after cataract surgery is usually routine. All our patients had been prescribed with topical steroid and moxifloxacin for at least 1 week after cataract surgery. One of them received IVI steroid during PPV due to persistent inflammation after six IVIs of amikacin. Topical steroid was prescribed for all nine patients for at least 1 month, depending on clinical course after IVI or PPV. Because steroid usage may lead to a prolonged course with a potentially critical outcome even under antibiotic coverage [10], its initiation and discontinuation should be evaluated prudently. The prolonged use of topical steroid as well as its use before the diagnosis of NTME may have accounted for the worse visual outcomes in our cases.
To the best of our knowledge, this is the largest report of a clustered outbreak of postoperative cataract endophthalmitis caused by the same strain of M. abscessus/chelonae. Inadequate sterilization of the surgical instruments, the use of contaminated balanced salt solution, or reused needles to extract medications may be the potential sources of infection. Irrigation fluid and environmental samples were obtained from the local ophthalmic clinic but with the culture results were negative. Although we were unable to prove it, the contaminated solutions used in the autoclave were most probably the source of this pathogen. Mycobacteria cannot be thoroughly killed with the usual disinfectants, and surgical equipment must be autoclaved. Complete autoclave sterilization before cataract surgery may help to prevent NTM infection.
The retrospective study design, small number of patients, and the lack of standardized treatment protocol are major limitations of our study.
Conclusions
The visual prognosis of patients with NTME is very poor even with aggressive treatment. Early diagnosis and proper management are necessary for a clustered outbreak of postoperative NTME. Standard perioperative disinfection such as autoclave sterilization of instruments, strict periocular sterilization procedures, sterile packing solution, and sterile eye drops may help in reducing such outbreaks. For eradication of nontuberculous mycobacterium, aggressive surgical intervention, multiple intravitreal injections with early IOL removal, and intravitreal and systemic antibiotics may be required, as demonstrated in the current study, and are recommended treatment for NTME, but the outcomes are often poor. Further studies should be conducted for a more effective treatment strategy for such refractory infections as a negative culture result does not necessarily imply a bacteria-free infection.
Summary
What was known before
-
Nontuberculous mycobacterium endophthalmitis induces severe and persistent intraocular inflammation with poor outcomes despite aggressive treatments.
What this study adds
-
Autoclave sterilization and perioperative disinfection may help in reducing iatrogenic clustered infection.
References
Roussel TJ, Stern WH, Goodman DF, Whitcher JP. Postoperative mycobacterial endophthalmitis. Am J Ophthalmol. 1989;107:403–6.
Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273–90.
Ambler JS, Meisler DM, Zakov ZN, Hall GS, Spech TJ. Endogenous Mycobacterium chelonae endophthalmitis. Am J Ophthalmol. 1989;108:338–9.
Moorthy RS, Valluri S, Rao NA. Nontuberculous mycobacterial ocular and adnexal infections. Surv Ophthalmol. 2012;57:202–35.
Paulose RM, Joseph J, Narayanan R, Sharma S. Clinical and microbiological profile of non-tuberculous mycobacterial endophthalmitis—experience in a tertiary eye care centre in Southern India. J Ophthalmic Inflamm Infect. 2016;6:27.
Hughes DS, Hill RJ. Infectious endophthalmitis after cataract surgery. Br J Ophthalmol. 1994;78:227.
Akçakaya AA, Sargın F, Erbil HH, Yaylalı SA, Mesçi C, Ergin S, et al. A cluster of acute-onset postoperative endophthalmitis over a 1-month period: investigation of an outbreak caused by uncommon species. Br J Ophthalmol. 2010;95:481–4.
Scott IU, Lieb DF, Flynn HW, Dessouki A, Murray TG, Miller D. Endophthalmitis caused by Mycobacterium chelonae: selection of antibiotics and outcomes of treatment. Arch Ophthalmol. 2003;121:573–6.
Shah M, Relhan N, Kuriyan AE, Davis JL, Albini TA, Pathengay A, et al. Endophthalmitis caused by nontuberculous Mycobacterium: clinical features, antimicrobial susceptibilities, and treatment outcomes. Am J Ophthal. 2016;168:150–6.
Kheir WJ, Sheheitli H, Abdul Fattah M, Hamam RN. Nontuberculous mycobacterial ocular infections: a systematic review of the literature. Biomed Res Int. 2015;2015:164989 https://doi.org/10.1155/2015/164989.
Lalitha P, Rathinam SR, Srinivasan M. Ocular infections due to non-tuberculous mycobacteria. Indian J Med Microbiol. 2004;22:231–7.
Venkateswaran N, Yeaney G, Chung M, Hindman HB. Recurrent nontuberculous mycobacterial endophthalmitis: a diagnostic conundrum. Clin Ophthalmol. 2014;8:837.
Girgis DO, Karp CL, Miller D. Ocular infections caused by non‐tuberculous mycobacteria: update on epidemiology and management. Clin Exp Ophthalmol. 2012;40:467–75.
Hung JH, Huang YH, Chang TC, Tseng SH, Shih MH, Wu JJ, et al. A cluster of endophthalmitis caused by Mycobacterium abscessus after cataract surgery. J Microbiol Immunol Infect. 2016;49:799–803.
El-Asrar AMA, Tabbara KF. Chronic endophthalmitis after extracapsular cataract extraction caused by Mycobacterium chelonae subspecies abscessus. Eye. 1995;9:798–801.
Ramaswamy AA, Biswas J, Bhaskar V, Gopal L, Rajagopal R, Madhavan HN. Postoperative Mycobacterium chelonae endophthalmitis after extracapsular cataract extraction and posterior chamber intraocular lens implantation. Ophthalmology. 2000;107:1283–6.
Mutyala S, Dieckert JP, Papasian CJ. Mycobacterium fortuitum endophthalmitis. Retina. 1996;16:122–4.
Wilhelmus KR. Nontuberculous mycobacterial endophthalmitis [comment]. Arch Ophthalmol. 2003;121:1663.
Marin-Casanova P, Calandria Amiguetti JL, Garcia-Martos P, Lozano Dominguez C, Hoyos Sanabria B, Puerto Alonso JL, et al. Endophthalmitis caused by Mycobacterium abscessus. Eur J Ophthalmol. 2003;13:800–2.
Matieli LC, De Freitas D, Sampaio J, Moraes NS, Yu MCZ, Hofling-Lima AL. Mycobacterium abscessus endophthalmitis: treatment dilemma and review of literature. Retina. 2006;26:826–9.
Palani D, Kulandai LT, Naraharirao MH, Guruswami S, Ramendra B. Application of polymerase chain reaction-based restriction fragment length polymorphism in typing ocular rapid-growing non-tuberculous mycobacterial isolates from three patients with postoperative endophthalmitis. Cornea. 2007;26:729–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hsu, CR., Chen, JT., Yeh, KM. et al. A cluster of nontuberculous mycobacterial endophthalmitis (NTME) cases after cataract surgery: clinical features and treatment outcomes. Eye 32, 1504–1511 (2018). https://doi.org/10.1038/s41433-018-0108-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0108-1
This article is cited by
-
Comparison of pain between bilateral ICL surgeries in patients with myopia
BMC Ophthalmology (2024)
-
Literature- and Experience-Based Consensus for Acute Post-operative Endophthalmitis and Endogenous Endophthalmitis in Taiwan
Ophthalmology and Therapy (2024)
-
Antimicrobial guide to posterior segment infections
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Nontuberculous mycobacterial endophthalmitis: case series and review of literature
BMC Infectious Diseases (2020)