Abstract
In a recent International Working Group on Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) study, prior arterial events and hypertension were predictors of subsequent arterial thrombosis whereas prior venous events and age ≥65 years predicted venous thrombosis in polycythemia vera (PV). In the current study, we sought to validate the above findings and identify additional predictors of arterial versus venous thrombosis. At a median follow up of 109 months, thrombosis after diagnosis occurred in 128 (22%) patients; 82 (14%) arterial and 57 (10%) venous events. On multivariate analysis, prior arterial events (<0.0001), hyperlipidemia (p = 0.03), and hypertension (p = 0.02) predicted subsequent arterial events. In comparison, prior venous events (p = 0.05), leukocytosis ≥11 × 109/L (p = 0.002), and major hemorrhage (p = 0.02) were predictors of subsequent venous events. Salient associations with arterial thrombosis included age ≥ 60 years, hypertension, diabetes, hyperlipidemia and normal karyotype whereas age ≤ 60 years, females, palpable splenomegaly and history of major hemorrhage were associated with venous thrombosis. TET2 or ASXL1 mutations did not impact arterial nor venous thrombosis. In conclusion, we identify distinct associations for arterial versus venous thrombosis in PV and confirm that a prior arterial or venous thrombotic event is the most reliable predictor of subsequent events.
Similar content being viewed by others
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm (MPN) characterized by clonal erythrocytosis resulting from constitutive activation of the Janus Kinase and Signal Transducer and Activator of Transcription (JAK-STAT) signal transduction pathway. The diagnosis of PV is based on the 2016 World Health Organization (WHO) criteria utilizing a composite assessment of clinical and laboratory features1. PV is associated with burdensome symptoms, reduced quality of life and thrombohemorrhagic complications with potential for myelofibrotic and/or leukemic transformation2. Thrombotic complications are a major cause of morbidity and mortality with a reported incidence of 12–39%3. Patients suffer from large vessel arterial and venous thrombosis, as well as microcirculatory symptoms such as dizziness, headaches, visual disturbances, erythromelalgia, distal paresthesia and acrocyanosis3. In the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) prospective study, age older than 65 years and prior thrombotic events were identified as risk factors for cardiovascular events among PV patients4. Importantly, arterial and venous thrombosis are two biologically different processes with distinct risk factors. The International Working Group on Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) identified prior arterial events and hypertension as risk factors for arterial thrombosis and prior venous events and age ≥ 65 years as risk factors for venous thrombosis in PV5. In this single center study, we sought to validate the findings of the IWG-MRT based on WHO 2016 PV diagnostic criteria, as well as identify additional risk factors for arterial versus venous thrombosis, including possible associations with the most frequent “non-driver” mutations: Tet methylcytosine dioxygenase 2 (TET2) (22%) and Additional sex combs like-1 (ASXL1) (12%)6.
Material and methods
The current study was approved by the institutional review board of Mayo Clinic (Rochester, MN). Study patients were selected from our institutional database of MPN and fulfilled the 2016 WHO criteria for the diagnosis of PV1. Clinical and laboratory data was obtained from the time of diagnosis. Only major thrombotic events were considered specifically: 1) Arterial thrombosis: acute myocardial infarction (MI), angina pectoris, ischemic stroke, transient ischemic attack (TIA), peripheral arterial disease (PAD) or 2) Venous thrombosis: pulmonary embolism (PE), deep vein thrombosis (DVT), portal or mesenteric thrombosis (splanchnic vein thrombosis), cerebral sinus vein thrombosis. Thrombotic events were objectively identified based on diagnostic imaging. Post-PV thrombotic events were defined as events occurring ≥ 4 weeks following PV diagnosis. Major hemorrhage was defined based on International Society on Thrombosis and Hemostasis (ISTH) definitions as: gastrointestinal, internal organ, intraarticular, cerebrovascular, retroperitoneal bleed or any bleeding requiring medical and/or surgical intervention, hospitalization and/or resulting in death. Therapy in the study included low dose Aspirin (81 mg PO once daily) and cytoreductive therapy including: hydroxyurea, busulfan, interferon, anagrelide, and JAK inhibitors. Cytogenetic analysis and reporting was done according to the International System for Human Cytogenetic Nomenclature7. In addition to JAK2V617F and exon 12 testing, mutation screening for TET2 and ASXL1 was performed according to conventional methods6,8. Differences in the distribution of continuous variables between categories were analyzed by either Mann–Whitney (for comparison of two groups) or Kruskal–Wallis test (comparison of three or more groups). Patient groups with nominal variables were compared by χ 2-test. Thrombosis-free survival (TFS) was determined from the time of diagnosis to the time of event occurrence after diagnosis (uncensored) or last contact/date of death (censored). All survival curves were prepared by the Kaplan–Meier methods and compared by the log-rank test. Cox proportional hazard regression model was applied to carry out multivariate analysis. We considered p-values less than 0.05 as significant. The Stat View (SAS Institute, Cary, NC, USA) statistical package was used for all calculations.
Results
Among a total of 587 patients, the median age of our cohort was 60 years (range; 17–94 years) with 48% males. Based on current European Leukemia Net (ELN) classification9, 64% were “high risk” PV (age ≥ 60 years and/or history of prior thrombosis). Patient characteristics are outlined in Table 1. The incidence of cardiovascular risk factors was as follows: hypertension (42%), diabetes (9%), hyperlipidemia (21%), and history of active or remote smoking (29%). As expected, the majority of patients were JAK2 mutated (99%) with 18 and 11% harboring TET2 and ASXL1 mutations, respectively. At a median follow up of 109 months, 14 and 4% of patients experienced myelofibrotic and leukemic transformation, respectively.
A total of 235 (40%) patients experienced any thrombotic event which included 153 (26%) arterial and 104 (18%) venous thromboses. One hundred forty-six patients (25%) had a thrombotic event prior to or at the time of diagnosis followed by 40 (27%) of patients with a recurrent event after PV diagnosis. Overall, post diagnosis, a total of 128 (22%) thrombotic events occurred with 11 (5%) of PV patients having experienced both an arterial and venous event. Table 2 outlines the type of arterial and venous events with acute coronary syndrome (ACS) being the most common arterial events (45%) experienced before or at time of diagnosis and cerebrovascular events occurring most commonly post-diagnosis (44%). In comparison, splanchnic vein thrombosis (45%) represented the most common venous events before or at diagnosis while deep venous thrombosis (DVT) (44%) were more common venous events post-diagnosis.
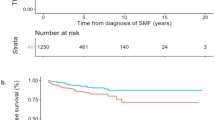
In multivariate analysis, prior thrombotic events (HR 1.9, CI: 1.2–2.9, p = 0.03) and leukocytosis (WBC ≥ 11 × 109/L) (HR 1.3, CI: 0.9–2.0, p = 0.03) were predictive of subsequent thrombotic events in general. Specifically, prior arterial events (HR 2.7, CI: 1.7–4.5, p < 0.0001) and hyperlipidemia (HR 1.8, CI: 1.1–2.9, p = 0.03) were predictors of subsequent arterial events. In contrast, presence of major hemorrhage at diagnosis (HR 3.4, CI: 1.2–9.7, p = 0.02), leukocytosis (WBC ≥ 11 × 109/L) (HR 2.0, CI: 1.1–3.8, p = 0.002) and prior venous events (HR 2.2, CI: 0.9–4.9, p = 0.05) were predictive of future venous events. Non-driver mutations (TET2 and ASXL1) did not significantly influence neither arterial nor venous events. Table 3 summarizes results from both univariate and multivariate analysis for thrombosis-free survival. Based on significant parameters obtained on multivariate analysis, thrombosis-free survival data are shown in Figs. 1 and 2. Given the limited number of thrombotic events after diagnosis, we further explored clinical associations with arterial and venous thrombotic events occurring anytime either at or after PV diagnosis. As shown in Tables 4 and 5, the following salient associations were noted with arterial and venous events. Older patients (≥60years) (p = 0.006), patients with hypertension (p = 0.004), diabetes (p = 0.005), hyperlipidemia (p < 0.0001) or with normal cytogenetics (p = 0.01) experienced higher rates of arterial thrombosis. In contrast, younger patients (≤60 years) (p = 0.0005), females (p = 0.008), patients with palpable splenomegaly (p = 0.006) and a history of major hemorrhage (p = 0.0002) were more likely to experience venous thrombotic events. Patients who experienced a venous event were less likely to have cardiovascular risk factors: hypertension, hyperlipidemia or be active smokers.
Discussion
In the current large series of PV patients that were evaluated and carefully followed at a single institution, we were able to systematically analyze the differences in risk factors for arterial versus venous thrombosis. In our study, the incidence of arterial (17%) and venous thrombosis (9%) before or at PV diagnosis, were similar to prior studies; ECLAP (27 and 11%, respectively), CYTO-PV (17 and 12%, respectively) and IWG-MRT study (16 and 7.4%, respectively)4,5,10,. Twenty-two percent of our patients experienced a thrombotic event after diagnosis (2.4%/year), which was also similar to the CYTO-PV and ECLAP studies (2.7/year and 2.6%/year, respectively), but lower than the IWG-MRT study (4.4%/year)4,5,10.
We confirm that a history of arterial and/or venous thrombosis is the most reliable risk factor for future arterial and venous events as shown in the IWG-MRT study5. A novel observation is the association of leukocytosis with increased risk of all thrombotic events, specifically venous thrombosis which differs from the findings of the ECLAP study in which leukocytosis ≥ 15 × 109/L was associated with increased cardiovascular events among PV patients4. Leukocytosis is also a well-established risk factor for thrombosis and inferior survival among essential thrombocytosis (ET) patients11,12,13.
The role of cardiovascular risk factors in thrombosis was explored, with hyperlipidemia having an impact on future arterial thrombosis. As expected, we identified older age (≥ 60 years), hypertension, diabetes, hyperlipidemia, and normal karyotype to be associated with increased risk of overall arterial thrombosis. In contrast, younger patients, females, patients with palpable splenomegaly and a history of major hemorrhage were more likely to experience a venous thrombotic event. The IWG-MRT had also identified this association of female gender and venous thrombosis which may be related to use of concomitant hormonal therapy but was not specifically evaluated in either study5. The association of splenomegaly and thrombosis has been evaluated in ET with conflicting results14,15.
In conclusion, our study reinforces the importance of a history of thrombosis in predicting future thrombotic events and supports that arterial and venous events are distinct entities with specific risk factors that require careful evaluation and management. Additionally, the non-driver TET2 and ASXL1 mutations did not impact arterial nor venous thrombosis. Further studies are needed to conclusively confirm the role of specific risk factors identified for arterial versus venous thrombosis.
References
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Tefferi, A. & Barbui, T. CME Information: polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 90, 163–173 (2015).
Elliott, M. A. & Tefferi, A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br. J. Hematol. 128, 275–290 (2005).
Marchioli, R. et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 23, 2224–2232 (2005).
Barbui, T. et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood 124, 3021–3023 (2014).
Tefferi, A. et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 1, 21–30 (2016). http://www.bloodadvances.org/lookup/doi/10.1182/bloodadvances.2016000216.
Simons, A., Shaffer, L. G. & Hastings, R. J. Cytogenetic nomenclature: changes in the ISCN 2013 compared to the 2009 edition. Cytogenet. Genome Res. 141, 1–6 (2013).
Patnaik M. M. et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia 28:2206–2212 (2014). http://www.nature.com/doifinder/10.1038/leu.2014.125.
Barbui, T. et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European leukemia. Net J. Clin. Oncol. 29, 761–770 (2011).
Marchioli, R. et al. Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl. J. Med. 368, 22–33 (2013). http://www.nejm.org/doi/abs/10.1056/NEJMoa1208500.
Wolanskyj, A. P., Schwager, S. M., McClure, R. F., Larson, D. R. & Tefferi, A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin. Proc. 81, 159–166 (2006). http://www.sciencedirect.com/science/article/pii/S0025619611616649.
Carobbio, A. et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 117, 5857–5859 (2011).
Palandri, F. et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: A retrospective study. Ann. Hematol. 90, 933–938 (2011).
Andriani, A. et al. Spleen enlargement is a risk factor for thrombosis in essential thrombocythemia: evaluation on 1,297 patients. Am. J. Hematol. 91, 318–321 (2016).
Haider, M., Gangat, N., Hanson, C. & Tefferi, A. Splenomegaly and thrombosis risk in essential thrombocythemia:the mayo clinic experience. Am. J. Hematol. 91, E296–E297 (2016).
Acknowledgements
This work was supported by the Mayo Clinic Rochester, Minnesota and the University of Calgary, Alberta.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cerquozzi, S., Barraco, D., Lasho, T. et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer Journal 7, 662 (2017). https://doi.org/10.1038/s41408-017-0035-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-017-0035-6
This article is cited by
-
Optimization of cardiovascular risk factor management in patients with BCR::ABL1 negative chronic myeloproliferative neoplasms, current knowledge, and perspectives
Annals of Hematology (2024)
-
Cancer-associated thrombosis in hematologic malignancies
International Journal of Hematology (2024)
-
Recurrent arterial thrombosis as the primary symptom in a patient with polycythemia vera
Annals of Hematology (2023)
-
JAK2V617F variant allele frequency, non-driver mutations, single-nucleotide variants and polycythemia vera outcome
Journal of Cancer Research and Clinical Oncology (2023)
-
Machine learning analyses constructed a novel model to predict recurrent thrombosis in adults with essential thrombocythemia
Journal of Thrombosis and Thrombolysis (2023)