Abstract
The literature on non-genetic peripheral biomarkers for major mental disorders is broad, with conflicting results. An umbrella review of meta-analyses of non-genetic peripheral biomarkers for Alzheimer’s disease, autism spectrum disorder, bipolar disorder (BD), major depressive disorder, and schizophrenia, including first-episode psychosis. We included meta-analyses that compared alterations in peripheral biomarkers between participants with mental disorders to controls (i.e., between-group meta-analyses) and that assessed biomarkers after treatment (i.e., within-group meta-analyses). Evidence for association was hierarchically graded using a priori defined criteria against several biases. The Assessment of Multiple Systematic Reviews (AMSTAR) instrument was used to investigate study quality. 1161 references were screened. 110 met inclusion criteria, relating to 359 meta-analytic estimates and 733,316 measurements, on 162 different biomarkers. Only two estimates met a priori defined criteria for convincing evidence (elevated awakening cortisol levels in euthymic BD participants relative to controls and decreased pyridoxal levels in participants with schizophrenia relative to controls). Of 42 estimates which met criteria for highly suggestive evidence only five biomarker aberrations occurred in more than one disorder. Only 15 meta-analyses had a power >0.8 to detect a small effect size, and most (81.9%) meta-analyses had high heterogeneity. Although some associations met criteria for either convincing or highly suggestive evidence, overall the vast literature of peripheral biomarkers for major mental disorders is affected by bias and is underpowered. No convincing evidence supported the existence of a trans-diagnostic biomarker. Adequately powered and methodologically sound future large collaborative studies are warranted.
Similar content being viewed by others
Introduction
One of the overarching goals of the emerging field of precision psychiatry is to incorporate advanced technologies to provide an objective data-driven personalized approach to the diagnosis and treatment of mental disorders1,2. However, unlike other medical fields, there is an acknowledged ‘translational gap’ in psychiatry1,3. In parallel, the field of biological psychiatry aiming to provide a neurobiological basis for current mental disorders, has provided contrasting results, even in pivotal biomarkers4. Hence, the diagnosis and clinical management of major mental disorders is still entirely based on psychopathological knowledge, while the treatment of mental disorders remains predominantly based on ‘trial and error’, albeit within the confines of fitting evidence-based prescription to a clinical profile5.
Over the past two decades the field has witnessed a remarkable increase in interest on biomarkers for mental disorders6. In particular, the literature on non-genetic peripheral biomarkers has grown exponentially, with the publication of several systematic reviews and meta-analyses7,8,9,10,11,12. The identification and validation of biomarkers for mental disorders are thought to be crucial steps in the development of precision and biological psychiatry, and its ultimate incorporation in the current landscape of psychiatric care is expected to follow1. However, this change is not translating into meaningful modifications in clinical practice.
Several reasons may contribute to the contrast between the overall volume of this literature and the limited applicability of peripheral biomarkers in current psychiatric practice. For instance, it has been proposed that conventional psychiatric diagnoses based, for example, on the Diagnostic and Statistical Manual for Mental Disorders (DSM) may lack biological validity2,13. In this respect, it has been proposed that similarly to genetic14 and neuroimaging15,16 biomarkers, alterations in peripheral biomarkers for major mental disorders may be shared across distinct diagnostic categories, and thus may have a transdiagnostic nature6. However, what is a trans-diagnostic construct in psychiatry remains debated, and no study has properly assessed the trans-diagnostic nature of any biomarker with a methodologically sound approach17.
In addition to the lack of consensus on how to define a trans-diagnostic construct, a core reason for this translational gap even in a single disorder may be due to the presence of several biases including large heterogeneity, an excess significance bias, as well as a selective reporting of statistically significant (i.e., ‘positive’) findings without proper adjustment to multiple confounders. An Umbrella review systematically evaluates and collects information from multiple systematic reviews and meta-analyses on all outcomes of a given topic for which these have been performed18. Umbrella reviews are particularly suited to uncover these biases19, as previously demonstrated with respect to peripheral biomarkers for depression20, bipolar disorder (BD)20, and schizophrenia21. However, those previous umbrella reviews have only addressed studies that have differentiated participants with a specific mental disorder and healthy controls, and not changes in peripheral biomarkers following treatment for these disorders. Moreover, those umbrella reviews focused on only one mental disorder each.
Thus, the current work provides a comprehensive umbrella review of meta-analyses of peripheral biomarkers for major mental disorders related to high prevalence and burden, namely Alzheimer’s disease (AD), autism spectrum disorder (ASD), BD, major depressive disorder (MDD), and schizophrenia, including also first-episode psychosis (FEP) stage. We aimed to re-assess the presence of bias in this literature and identify biomarkers that would be supported by most convincing evidence. In addition, we aimed to identify shared and unique alterations in biomarkers for those major mental disorders among those supported by either convincing or highly suggestive evidence. In the current analysis, we considered both studies that investigated abnormalities in peripheral biomarkers of mental disorders compared to controls (i.e., between-group meta-analyses) and ones that assessed alterations in the levels of peripheral biomarkers after treatment (i.e., within-group meta-analyses).
Methods
Literature search
We conducted an umbrella review, which is a systematic collection of multiple systematic reviews and meta-analyses done in a specific research topic22. The PubMed/MEDLINE database was searched from inception to February 17, 2019 for all available meta-analyses non-genetic peripheral biomarkers for major mental disorders. This search strategy was augmented through (1) handsearching the reference lists of included articles and (2) tracking citations of included articles through the Google Scholar database. The search string used in the current umbrella review was developed by a professional librarian and is available in the Supplementary Online material. The searches, screening, data extraction, and methodological quality appraisal were independently conducted by at least two investigators. Disagreements were resolved through consensus. When a consensus could not be reached a third investigator (AFC) made the final decision. An a priori defined protocol was followed (available upon reasonable request to the corresponding author of the current manuscript).
Eligibility criteria
We included meta-analyses published in peer-reviewed journals that assessed and synthesized studies on peripheral biomarkers for adults with AD, ASD, BD, MDD, Schizophrenia, including FEP. We included studies in which biomarkers were assayed in participants with a specific mental disorder compared to controls (i.e., between-group meta-analyses), as well as ones which assessed changes in peripheral biomarkers in any of those disorders after treatment (i.e., within-group meta-analyses). Studies published in English were considered for inclusion. This decision was made because most well-designed systematic reviews and meta-analyses are published in English. We included studies in which diagnoses of mental disorders were conducted by means of a validated structured interview based on standard diagnostic criteria such as the International Classification of Disease (ICD) or the Diagnostic and Statistical Manual of Mental Disorders (DSM). We also considered studies in which a probable diagnosis of a major depressive episode was established through a validated screening questionnaire as well as studies in which a diagnosis of FEP was based on clinical assessment by a mental health care provider. We excluded the following types of studies: (1) systematic reviews without a meta-analytic synthesis of the evidence; (2) animal studies; (3) studies of other types of biomarkers (for example, genetic biomarkers); (4) studies that included participants with two or more diagnoses; (5) studies that included participants with other primary psychiatric diagnoses (e.g. anxiety disorders); (6) studies that investigated biomarkers for other purposes (for example, biomarkers of risk, stage or prognosis)23; (7) studies conducted in pediatric samples (except from ASD and FEP); and (8) if there was more than one meta-analysis for the same biomarker in the same population, we considered only the largest MA (i.e., the one with the largest number of included individual studies).
Data extraction
For each eligible reference, we extracted the first author, year of publication, specific diagnoses assessed, as well as the number of included studies. We also extracted the summary effect size (ES) measure of each meta-analysis considering the ES used in each study. When available, the following variables were extracted at a study-level: number of cases, number of controls, sample size, ES, and study design. In each eligible reference, we only included the primary analyses due to the expected large amount of evidence. However, when included references provided details on the mood state of participants (e.g. mania or bipolar depression), we also extracted this information at an individual-study level.
Statistical analysis and methodological quality appraisal
Data were analyzed from March 1, 2019 to October 10, 2019. We estimated ESs and 95% confidence intervals (CIs) using both fixed and random-effects modeling24. Due to the anticipated high heterogeneity observed in meta-analyses of peripheral biomarkers for major mental disorders, random-effects calculations were considered in this review. When ESs were not provided as standardized mean difference (SMD) metrics (e.g., odds ratio), we converted the primary ESs to SMD25. We also estimated the 95% prediction interval, which accounts for between-study heterogeneity and assesses the uncertainty of the effect that would be expected in a new study addressing the same association26. For the largest study included in each meta-analytic estimate, we calculated the standard error (SE) of the ES. If the SE of the ES is <0.1, then the 95% CI will be <0.20 (i.e., less than the magnitude of a small ES). We calculated the I2 metric to quantify between-study heterogeneity. Values ≥50% and ≥75% are indicative of large and very large heterogeneity, respectively27. To assess evidence of small-study effects, we used the asymmetry test developed by Egger et al. 28. A P-value <0.10 in the Egger’s test and the ES of the largest study being more conservative than the summary random-effects ES of the meta-analysis were considered indicative of small-study effects20. We also annotated whether the association reported in each meta-analytic estimate was nominally significant at a P < 0.05 level as well as at a P < 0.005 level. The level of P < 0.005 has been proposed as a more stringent level of significance that could increase the reproducibility of many fields29.
We also determined whether the meta-analysis had a statistical power ≥ 80% to detect either a small (i.e., ES ≥ 0.2) or a medium (i.e., ES ≥ 0.5). We used the method described in detail elsewhere30. Finally, we also assessed evidence of excess of significance bias with the Ioannidis test31. Briefly, this test estimates whether the number of studies with nominally significant results (i.e., P < 0.05) among those included in a meta-analysis is too large considering their power to detect significant effects at an alpha level of 0.05. First, the power of each study is estimated with a non-central t distribution. The sum of all power estimates provides the expected (E) number of datasets with nominal statistical significance. The actual observed (O) number of statistically significant datasets is then compared to the E number using a χ2-based test31. Since the true ES of a meta-analysis cannot be precisely determined, we considered the ES of the largest dataset as the plausible true ES. This decision was based on the fact that simulations indicate that the most appropriate assumption is the ES of the largest dataset included in the meta-analysis32. Excess significance for a single meta-analysis was considered if P < 0.10 in Ioannidis’s test and O > E20. We graded the credibility of each association according to the following categories: convincing (class I), highly suggestive (class II), suggestive (class III), weak evidence (class IV), and non-significant associations (Table S1).
For evidence supported by either class I or class II evidence, we used credibility ceilings, which is which is a method of sensitivity analyses to account for potential methodological limitations of observational studies that might lead to spurious precision of combined effect estimates. In brief, this method assumes that every observational study has a probability c (credibility ceiling) that the true ES is in a different direction from the one suggested by the point estimate33. The pooled ESs were estimated considering a wide range of credibility ceilings. All analyses were conducted in STATA/MP 14.0 (StataCorp, USA) with the metan package.
The methodological quality of included systematic reviews and meta-analyses was also appraised using the Assessment of Multiple Systematic Reviews (AMSTAR) instrument, which has been validated for this purpose34,35. Scores range from 0 to 11 with higher scores indicating greater quality. The AMSTAR tool involves dichotomous scoring (i.e. 0 or 1) of 11 items related to assess methodological rigor of systematic reviews and meta-analyses (e.g., comprehensive search strategy, publication bias assessment). AMSTAR scores are graded as high (8–11), medium (4–7) and low quality (0–3)34.
Results
Our search strategy identified 1161 unique references of which 991 were excluded after title/abstract screening and 170 underwent full-text review (Fig. 1). Therefore, 110 references met inclusion criteria7,8,9,10,11,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139, and 60 references were excluded with reasons (Table S2). In the 110 included references, there were 81 between-group meta-analytic estimates for MDD, 79 for AD, 62 for schizophrenia, 45 for ASD, 37 for BD, and 15 for FEP. In addition, there were 25 within-group meta-analytic estimates for MDD, 13 for Schizophrenia, and 2 for BD (Mania) (Table S3). In total, there were 247,678 biomarker measurements estimates in cases and 476,340 assays in controls across between-group meta-analyses, while there were 9298 biomarker measurements across within-group meta-analytic estimates (Table S3). One hundred and ninety meta-analytic estimates were statistically significant at a P-value < 0.05, whilst 109 were significant at a P-value < 0.005 (Table S3).
Power of meta-analyses
Fifteen between-group meta-analytic estimates had an estimated power >0.8 to detect a small ES, and 145 meta-analyses (126 between-group meta-analyses) had an estimated power >0.8 to detect a medium ES (Table S3).
Heterogeneity and prediction intervals
No evidence of large heterogeneity (i.e., I2 < 50%) was found in 65 meta-analyses (18.1%), whilst 294 (81.9%) meta-analytic estimates had evidence of large heterogeneity (i.e., I2 > 50%). The prediction interval crossed the null value in 341 (94.9%) meta-analytic associations, while prediction intervals of 20 (5.0%) meta-analyses did not cross the null value (Table S3).
Small-study effects and excess significance bias
Evidence of small-study effects, which is an indication of publication bias, was observed in 38 (10.6%) meta-analyses, whilst evidence of excess of significance bias was verified in 74 (20.6%) meta-analytic estimates (Tables S3).
Grading of the evidence
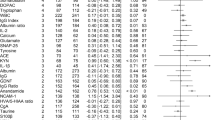
Only 2 (0.5%) meta-analytic estimates exhibited class I evidence (83, 119). In euthymic BD participants there was an increase in basal cortisol awakening levels (Hedges’g = 0.25; 95% CI: 0.15–0.35, P < 0.005) compared to controls87. Participants with schizophrenia presented decreased Vitamin B6 (pyridoxal) levels relative to controls123. In addition, 42 (11.7%) meta-analytic estimates were supported by class II evidence, of which 3 were derived from within-group meta-analyses (Table 1). Among those estimates, C-reactive protein levels were increased in euthymic BD, bipolar mania, and in MDD relative to controls80,102. In addition, soluble interleukin-(IL)-2 receptor (sIL-2R) levels were increased in MDD and in schizophrenia relative to controls7,8. Moreover, levels of antibodies against the N-methyl-d-aspartate receptor (NMDA-R) were elevated in BD and in schizophrenia relative to controls85. Brain-derived neurotrophic factor (BDNF) levels were decreased in AD and in MDD44,110. Furthermore, levels of insulin-like growth factor-1 (IGF-1) were elevated in bipolar mania and in MDD relative to controls84. The remaining findings supported by type II evidence were unique to a single disorder (Table 1).
Of the 44 biomarkers supported by either type I or type II evidence, 37 (84.1%) survived 10% credibility ceilings (Table 2).
Qualitative methodological appraisal of eligible meta-analyses
Qualitative methodological appraisal of eligible meta-analyses through the AMSTAR tool revealed that 49 references were classified as high, 58 as medium, and 3 as low methodological quality, respectively (Table S4). The overall methodological quality of included references was high according to the AMSTAR [(median: 8; IQR = 2 (7–9)] (Table S4).
Discussion
Our umbrella review provided an up-dated synthesis of the literature of non-genetic peripheral biomarkers for major mental disorders. We included data from 733,316 biomarker measurements. However, in this vast literature only two associations met a priori defined criteria for convincing evidence, whilst 42 meta-analytic estimates met criteria for highly suggestive evidence. This collaborative effort found compelling evidence that overall the literature on non-genetic peripheral biomarkers has a high prevalence of different types of bias. In addition, this umbrella review provides relevant insights for the conduct of further studies to investigate the associations supported by most convincing evidence. It should also be noted that overall the methodological quality of eligible meta-analyses as assessed with the AMSTAR tool was high, which provides further credibility to our quantitative grading of findings.
Associations supported by convincing evidence merit discussion. First, euthymic participants with BD exhibited a high cortisol awakening response relative to controls87. This finding indicates that the hypothalamic–pituitary–adrenal (HPA) axis is disrupted in BD on a trait-like basis. This suggests that the HPA axis could be targeted in BD140 to improve cognitive function, which may be compromised even during euthymic states141,142. In addition, participants with schizophrenia exhibited decreased vitamin B6 (pyridoxal) levels compared to controls123. This suggests that individuals with schizophrenia may present aberrations in the one-carbon cycle where pyridoxal is a main metabolic component. An alternative explanation might be the poor nutrition which frequently affects people with schizophrenia98. This finding is consistent with a recent systematic review and meta-analysis which provided preliminary evidence that adjunctive pharmacological interventions targeting the one-carbon cycle may improve negative symptoms in schizophrenia (although the clinical significance of this improvement may remain questionable143 and aligns with recent evidence showing that adjunctive treatment with B-vitamins may improve symptomatic outcomes in treatment of psychotic disorders144,145).
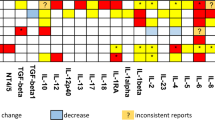
Importantly, only five biomarkers were found to be significantly associated with more than one mental disorder. Also, the highest class of evidence for these biomarkers was II. Moreover, no study applied a methodologically solid approach to assess the trans-diagnostic nature of any biomarker17. We found peripheral elevation on the acute phase reactant, CRP, in BD (both during euthymia and mania) as well as in MDD providing evidence that these disorders are at least partly associated with peripheral inflammation. In addition, the s-IL-2R was increased in both MDD and schizophrenia relative to controls. It is noteworthy that IL-2 is a key cytokine involved in the development, survival and function of regulatory T cells (TRegs)146,147, and it has been recently proposed that aberrations in “fine tuning” immune-regulatory mechanisms may contribute to the pathophysiology of both MDD and schizophrenia148,149. Antibodies against the NMDA-R were increased in BD and schizophrenia. This finding is consistent with the existence of autoantibodies against the GluN1 subunit of this receptor in patients with psychotic manifestations150,151. Furthermore, lower serum BDNF levels were observed in participants with MDD and AD relative to controls. This finding is consistent with the “neurotrophic hypothesis” of depression152, while parallel lines of evidence suggest that aberrations in BDNF signaling may contribute to neurodegeneration in AD153. Finally, lower levels of IGF-1 were observed in bipolar mania and MDD compared to controls. This finding is consistent with the modulatory role of glucose-related signaling including the trophic molecule IGF-1 in hippocampal plasticity154. In addition, preclinical evidence suggests that IGF-1 may be involved in the pathophysiology of affective disorders155,156.
There is an emerging body of literature investigating the putative role of non-genetic peripheral biomarkers for the prediction of treatment response in major mental disorders. Surprisingly, no such biomarkers met criteria for convincing evidence, while only three biomarkers met criteria for type II evidence. Adiponectin levels in schizophrenia decreased after treatment with second-generation antipsychotics. This is an interesting finding since hypoadiponectinemia has been associated with a wide range metabolic diseases which are common untoward effects of these drugs157,158. In addition, IL-6 levels decreased after treatment with antidepressants. These data are consistent with preclinical findings which show that antidepressants have anti-inflammatory properties and may also inhibit M1 microglia polarization159. Finally, lipid peroxidation markers increased after antidepressant drug treatment for MDD.
It is worth noting that only 15 meta-analytic estimates had a power >0.80 to detect a small ES. In addition, previous umbrella reviews indicate that the vast majority of peripheral biomarker studies are substantially underpowered20. This may undermine the progress and reliability of this particular field and of neuroscience in general through the generation of spurious findings160. The “true” ESs of most non-genetic peripheral biomarkers may be expected to be small, similarly to those reported in the genetic literature. Therefore, the design of large, multicenter studies with an open pre-registered protocol, or the creation of Consortia, may be a crucial step to assess the role of peripheral biomarkers in the diagnosis and treatment of major mental disorders within the framework of precision psychiatry1, as the model adopted by the Enigma neuroimaging group161, or similarly to other large collaborative initiatives162. Likewise the creation of biomarker scores using a similar rationale as for the generation of polygenic risk scores may ultimately be a next step in this field.
Strengths and limitations
It should also be noted that large statistical heterogeneity was verified in most included meta-analytic estimates (81.9%). Although this is considered a relevant indicator of bias in this literature, it may also reflect genuine heterogeneity, which may occur both within and between major diagnostic categories163. In addition, methodological differences of individual studies included in the assessed meta-analyses may also contribute to heterogeneity. Those include, for example, the time of sample selection as well as measurement properties of the assays (e.g. intra-assay and inter-assay coefficients of variation). Guidelines to standardize the collection and measurement of peripheral biomarkers in psychiatry have been recently proposed164. Furthermore, differences in sample selection across individual studies might have contributed to the observed heterogeneity in some meta-analytic estimates. For example, illness stage and disorders in which mixed presentations are common (e.g., bipolar disorder) might have contributed to heterogeneity across some included meta-analyses. In addition, approaches to subtype major mental disorders according to frameworks such as the NIMH Research Domain Criteria may help to decrease the heterogeneity of this literature in the future through the study of biologically valid and more homogenous phenotypes13,163,165.
Conclusion
This umbrella review of non-genetic peripheral biomarkers for major mental disorders revealed that this literature is fraught with several biases and is underpowered. Nevertheless, two associations supported by convincing evidence and 42 associations supported by highly suggestive evidence were verified. Most associations supported by either convincing or highly suggestive evidence pertained to a single disorder. Future multi-centric studies with a priori publicly available protocols, with an ad-hoc methodology to assess the trans-diagnostic nature of biomarkers17, as well as the subtyping of these disorders into more biologically valid phenotypes, and enough statistical power may improve the reliability and reproducibility of this field, which is of relevance for the translation of biological and precision psychiatry into practice.
Code availability
Computer codes used in the analyses of the data are available after reasonable request to the corresponding author of the current study.
References
Fernandes, B. S. et al. The new field of ‘precision psychiatry’. BMC Med. 15, 80 (2017).
Insel, T. R. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. The. Am. J. Psychiatry 171, 395–397 (2014).
Collins, P. Y. et al. Grand challenges in global mental health. Nature 475, 27–30 (2011).
Fusar-Poli, P. & Meyer-Lindenberg, A. Forty years of structural imaging in psychosis: promises and truth. Acta Psychiatr. Scand. 134, 207–224 (2016).
Leucht, S., Hierl, S., Kissling, W., Dold, M. & Davis, J. M. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br. J. Psychiatry 200, 97–106 (2012).
Pinto, J. V., Moulin, T. C. & Amaral, O. B. On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: a systematic review. Neurosci. Biobehav. Rev. 83, 97–108 (2017).
Goldsmith, D. R., Rapaport, M. H. & Miller, B. J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709 (2016).
Kohler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 135, 373–387 (2017).
Kohler, C. A. et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55, 4195–4206 (2018).
Lai, K. S. P. et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 88, 876–882 (2017).
Masi, A. et al. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry 20, 440–446 (2015).
Brown, N. C., Andreazza, A. C. & Young, L. T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 218, 61–68 (2014).
Cuthbert, B. N. & Insel, T. R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126 (2013).
Gandal, M. J. et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697 (2018).
Zhang, B. et al. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 10, 920–939 (2016).
McTeague, L. M. et al. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry 174, 676–685 (2017).
Fusar-Poli, P. et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry 18, 192–207 (2019).
Papatheodorou, S. Umbrella reviews: what they are and why we need them. Eur. J. Epidemiol. 34, 543–546 (2019).
Fusar-Poli, P. & Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 21, 95–100 (2018).
Carvalho, A. F. et al. Bias in peripheral depression biomarkers. Psychother. Psychosom. 85, 81–90 (2016).
Belbasis, L. et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyse. Acta Psychiatr. Scand. 137, 88–97 (2018).
Ioannidis, J. P. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can. Med. Assoc. J. = J. l’Assoc. Med. Can. 181, 488–493 (2009).
Davis, J. et al. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol. Psychiatry 20, 152–153 (2015).
Lau, J., Ioannidis, J. P. & Schmid, C. H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 127, 820–826 (1997).
Polanin, J. R. & Snilstveit, B. Converting between effect sizes. Campbell Syst. Rev. 12, 1–13 (2016).
IntHout, J., Ioannidis, J. P., Rovers, M. M. & Goeman, J. J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6, e010247 (2016).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. ed.) 327, 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. ed.) 315, 629–634 (1997).
Benjamin, D. J. et al. Redefine statistical significance. Nat. Hum. Behav. 2, 6–10 (2018).
Hedges, L. V. & Pigott, T. D. The power of statistical tests in meta-analysis. Psychol. Methods 6, 203–217 (2001).
Ioannidis, J. P. & Trikalinos, T. A. An exploratory test for an excess of significant findings. Clin. Trials (Lond., Engl.) 4, 245–253 (2007).
Ioannidis. Clarifications on the application and interpretation of the test for excess significance and its extensions. J. Math. Psychol. 57, 84–187 (2013).
Papatheodorou, S. I., Tsilidis, K. K., Evangelou, E. & Ioannidis, J. P. Application of credibility ceilings probes the robustness of meta-analyses of biomarkers and cancer risk. J. Clin. Epidemiol. 68, 163–174 (2015).
Shea, B. J. et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 7, 10 (2007).
Shea, B. J. et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 62, 1013–1020 (2009).
Mullan, K., Cardwell, C. R., McGuinness, B., Woodside, J. V. & McKay, G. J. Plasma antioxidant status in patients with Alzheimer’s disease and cognitively intact elderly: a meta-analysis of case-control studies. J. Alzheimer’s Dis. 62, 305–317 (2018).
Xu, L. et al. Circulatory levels of toxic metals (aluminum, cadmium, mercury, lead) in patients with Alzheimer's disease: a quantitative meta-analysis and systematic review. J. Alzheimer’s Dis. 62, 361–372 (2018).
Shi, Y., Gu, L., Alsharif, A. A. & Zhang, Z. The distinction of amyloid-beta protein precursor (AbetaPP) ratio in platelet between Alzheimer’s disease patients and controls: a systematic review and meta-analysis. J. Alzheimer’s Dis. 59, 1037–1044 (2017).
de Wilde, M. C., Vellas, B., Girault, E., Yavuz, A. C. & Sijben, J. W. Lower brain and blood nutrient status in Alzheimer’s disease: results from meta-analyses. Alzheimer's Dement. 3, 416–431 (2017).
Annweiler, C., Llewellyn, D. J. & Beauchet, O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s Dis. 33, 659–674 (2013).
Song, F. et al. Meta-analysis of plasma amyloid-beta levels in Alzheimer’s disease. J. Alzheimer’s Dis. 26, 365–375 (2011).
Wang, C. et al. Meta-analysis of peripheral blood apolipoprotein E levels in Alzheimer’s disease. PLoS ONE 9, e89041 (2014).
Shanthi, K. B., Krishnan, S. & Rani, P. A systematic review and meta-analysis of plasma amyloid 1-42 and tau as biomarkers for Alzheimer’s disease. SAGE Open Med. 3, 2050312115598250 (2015).
Du, Y. et al. Postmortem brain, cerebrospinal fluid, and blood neurotrophic factor levels in Alzheimer’s disease: a systematic review and meta-analysis. J. Mol. Neurosci. 65, 289–300 (2018).
Yang, C. et al. Association between clusterin concentration and dementia: a systematic review and meta-analysis. Metab. Brain Dis. 34, 129–140 (2019).
Li, D. D., Zhang, W., Wang, Z. Y. & Zhao, P. Serum copper, zinc, and iron levels in patients with Alzheimer’s disease: a meta-analysis of case-control studies. Front. Aging Neurosci. 9, 300 (2017).
Schneider, L. S., Hinsey, M. & Lyness, S. Plasma dehydroepiandrosterone sulfate in Alzheimer’s disease. Biol. Psychiatry 31, 205–208 (1992).
Xu, J., Xia, L. L., Song, N., Chen, S. D. & Wang, G. Testosterone, estradiol, and sex hormone-binding globulin in Alzheimer’s disease: a meta-analysis. Curr. Alzheimer Res. 13, 215–222 (2016).
Lopes da Silva, S. et al. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimer’s Dement. 10, 485–502 (2014).
Squitti, R. et al. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s disease. J. Alzheimer’s Dis. 38, 809–822 (2014).
Du, N. et al. Inverse association between serum uric acid levels and Alzheimer’s disease risk. Mol. Neurobiol. 53, 2594–2599 (2016).
Inoshita, M. et al. A significant causal association between C-reactive protein levels and schizophrenia. Sci. Rep. 6, 26105 (2016).
Hu, X., Yang, Y. & Gong, D. Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in Alzheimer’s disease: a meta-analysis. Neurol. Sci. 37, 1671–1677 (2016).
Zhou, F. & Chen, S. Effects of gender and other confounding factors on leptin concentrations in Alzheimer’s disease: evidence from the combined analysis of 27 case-control studies. J. Alzheimer’s Dis. 62, 477–486 (2018).
Schrag, M. et al. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol. Dis. 59, 100–110 (2013).
Du, K., Liu, M., Pan, Y., Zhong, X. & Wei, M. Association of serum manganese levels with Alzheimeras disease and mild cognitive impairment: a systematic review and meta-analysis. Nutrients 9, pii: E231, https://doi.org/10.3390/nu9030231 (2017).
Liu, D. et al. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: a meta-analysis. Neurosci. Lett. 686, 10–16 (2018).
Ho, R. C. et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am. J. Geriatr. Psychiatry 19, 607–617 (2011).
Shen, L. & Ji, H. F. Associations between homocysteine, folic acid, vitamin B12 and Alzheimer’s disease: insights from meta-analyses. J. Alzheimer’s Dis. 46, 777–790 (2015).
Beydoun, M. A. et al. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14, 643 (2014).
Ventriglia, M. et al. Zinc in Alzheimer’s disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 46, 75–87 (2015).
Frustaci, A. et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic. Biol. Med. 52, 2128–2141 (2012).
Zhu, G. et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 9, 2153–2168 (2016).
Gabriele, S., Sacco, R. & Persico, A. M. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 24, 919–929 (2014).
Saghazadeh, A. & Rezaei, N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 79, 340–368 (2017).
Mazahery, H. et al. Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: systematic review and meta-analysis of case-control and randomised controlled trials. Nutrients 9, pii: E155, https://doi.org/10.3390/nu9020155 (2017).
Saghazadeh, A. & Rezaei, N. Brain-derived neurotrophic factor levels in autism: a systematic review and meta-analysis. J. Autism Dev. Disord. 47, 1018–1029 (2017).
Jafari, T., Rostampour, N., Fallah, A. A. & Hesami, A. The association between mercury levels and autism spectrum disorders: a systematic review and meta-analysis. J. Trace Elem. Med. Biol. 44, 289–297 (2017).
Main, P. A., Angley, M. T., O’Doherty, C. E., Thomas, P. & Fenech, M. The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: a systematic review and meta-analysis. Nutr. Metab. 9, 35 (2012).
Tseng, P. T. et al. Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutr. Res. (N. Y., NY) 50, 44–52 (2018).
Zheng, Z., Zhu, T., Qu, Y. & Mu, D. Blood glutamate levels in autism spectrum disorder: a systematic review and meta-analysis. PLoS ONE 11, e0158688 (2016).
Looney, S. W. & el-Mallakh, R. S. Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depression Anxiety 5, 53–65 (1997).
Rao, S. et al. Peripheral blood nerve growth factor levels in major psychiatric disorders. J. Psychiatr. Res. 86, 39–45 (2017).
Babaknejad, N., Sayehmiri, F., Sayehmiri, K., Mohamadkhani, A. & Bahrami, S. The relationship between zinc levels and autism: a systematic review and meta-analysis. Iran. J. Child Neurol. 10, 1–9 (2016).
Ogyu, K. et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 90, 16–25 (2018).
Lin, P. Y., Huang, S. Y. & Su, K. P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 68, 140–147 (2010).
Mokhtari, M., Arfken, C. & Boutros, N. The DEX/CRH test for major depression: a potentially useful diagnostic test. Psychiatry Res. 208, 131–139 (2013).
Petridou, E. T. et al. Folate and B12 serum levels in association with depression in the aged: a systematic review and meta-analysis. Aging Ment. Health 20, 965–973 (2016).
Zorn, J. V. et al. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36 (2016).
Fernandes, B. S. et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 3, 1147–1156 (2016).
Bartoli, F., Crocamo, C., Mazza, M. G., Clerici, M. & Carra, G. Uric acid levels in subjects with bipolar disorder: a comparative meta-analysis. J. Psychiatr. Res. 81, 133–139 (2016).
Tseng, P. T. et al. State-dependent increase in the levels of neurotrophin-3 and neurotrophin-4/5 in patients with bipolar disorder: a meta-analysis. J. Psychiatr. Res. 79, 86–92 (2016).
Rutigliano, G. et al. Peripheral oxytocin and vasopressin: biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 241, 207–220 (2016).
Tu, K. Y. et al. Significantly higher peripheral insulin-like growth factor-1 levels in patients with major depressive disorder or bipolar disorder than in healthy controls: a meta-analysis and review under guideline of PRISMA. Medicine 95, e2411 (2016).
Pearlman, D. M. & Najjar, S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr. Res. 157, 249–258 (2014).
Fernandes, B. S. et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 13, 289 (2015).
Belvederi Murri, M. et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology 63, 327–342 (2016).
Nascimento, K. K., Silva, K. P., Malloy-Diniz, L. F., Butters, M. A. & Diniz, B. S. Plasma and cerebrospinal fluid amyloid-beta levels in late-life depression: a systematic review and meta-analysis. J. Psychiatr. Res. 69, 35–41 (2015).
Romeo, B., Choucha, W., Fossati, P. & Rotge, J. Y. Meta-analysis of central and peripheral gamma-aminobutyric acid levels in patients with unipolar and bipolar depression. J. Psychiatry Neurosci. 43, 58–66 (2018).
Lin, P. Y. & Tseng, P. T. Decreased glial cell line-derived neurotrophic factor levels in patients with depression: a meta-analytic study. J. Psychiatr. Res. 63, 20–27 (2015).
Inoshita, M. et al. Elevated peripheral blood glutamate levels in major depressive disorder. Neuropsychiatr. Dis. Treat. 14, 945–953 (2018).
You, H. J., Cho, S. E., Kang, S. G., Cho, S. J. & Na, K. S. Decreased serum magnesium levels in depression: a systematic review and meta-analysis. Nord. J. Psychiatry 72, 534–541 (2018).
Mazza, M. G. et al. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 84, 229–236 (2018).
Shin, J. Y., Suls, J. & Martin, R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Ann. Behav. Med. 36, 33–43 (2008).
Bartoli, F. et al. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: a meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci. 268, 119–127 (2018).
Anglin, R. E., Samaan, Z., Walter, S. D. & McDonald, S. D. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br. J. Psychiatry 202, 100–107 (2013).
Swardfager, W. et al. Zinc in depression: a meta-analysis. Biol. Psychiatry 74, 872–878 (2013).
Firth, J. et al. Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophrenia Bull. 44, 1275–1292 (2018).
Chaumette, B. et al. Salivary cortisol in early psychosis: new findings and meta-analysis. Psychoneuroendocrinology 63, 262–270 (2016).
Pillinger, T. et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74, 261–269 (2017).
Hoen, W. P. et al. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 207, 1–12 (2013).
Haapakoski, R., Mathieu, J., Ebmeier, K. P., Alenius, H. & Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215 (2015).
Ni, M., You, Y., Chen, J. & Zhang, L. Copper in depressive disorder: a systematic review and meta-analysis of observational studies. Psychiatry Res. 267, 506–515 (2018).
Ciufolini, S., Dazzan, P., Kempton, M. J., Pariante, C. & Mondelli, V. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neurosci. Biobehav. Rev. 47, 359–368 (2014).
Wang, D., Zhai, J. X. & Liu, D. W. Serum folate levels in schizophrenia: a meta-analysis. Psychiatry Res. 235, 83–89 (2016).
Fernandes, B. S. et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatry 21, 554–564 (2016).
Tseng, P. T., Cheng, Y. S., Chen, Y. W., Wu, C. K. & Lin, P. Y. Increased levels of vascular endothelial growth factor in patients with major depressive disorder: A meta-analysis. Eur. Neuropsychopharmacol. 25, 1622–1630 (2015).
Zhu, G. et al. Serum DHEAS levels are associated with the development of depression. Psychiatry Res. 229, 447–453 (2015).
Flatow, J., Buckley, P. & Miller, B. J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 74, 400–409 (2013).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol. Psychiatry 19, 791–800 (2014).
Wu, C. K., Tseng, P. T., Chen, Y. W., Tu, K. Y. & Lin, P. Y. Significantly higher peripheral fibroblast growth factor-2 levels in patients with major depressive disorder: a preliminary meta-analysis under MOOSE guidelines. Medicine 95, e4563 (2016).
Persons, J. E. & Fiedorowicz, J. G. Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J. Affect. Disord. 206, 55–67 (2016).
Ogawa, S. et al. Plasma l-tryptophan concentration in major depressive disorder: new data and meta-analysis. J. Clin. Psychiatry 75, e906–e915 (2014).
Greenhalgh, A. M. et al. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naive patients with nonaffective psychosis. Schizophrenia Res. 179, 57–63, https://doi.org/10.1016/j.schres.2016.09.026 (2017).
Chen, Y. W. et al. Significantly lower nerve growth factor levels in patients with major depressive disorder than in healthy subjects: a meta-analysis and systematic review. Neuropsychiatr. Dis. Treat. 11, 925–933 (2015).
Fernandes, B. S. et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatry 20, 1108–1119 (2015).
Aleksovska, K. et al. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS ONE 9, e106342 (2014).
Lachance, L. R. & McKenzie, K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis. Schizophrenia Res. 152, 521–527 (2014).
Berger, M. et al. Cortisol awakening response in patients with psychosis: systematic review and meta-analysis. Neurosci. Biobehav. Rev. 68, 157–166 (2016).
Plitman, E. et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophrenia Bull. 43, 764–777 (2017).
Stubbs, B., Wang, A. K., Vancampfort, D. & Miller, B. J. Are leptin levels increased among people with schizophrenia versus controls? A systematic review and comparative meta-analysis. Psychoneuroendocrinology 63, 144–154 (2016).
Qin, X. Y., Wu, H. T., Cao, C., Loh, Y. P. & Cheng, Y. A meta-analysis of peripheral blood nerve growth factor levels in patients with schizophrenia. Mol. Psychiatry 22, 1306–1312 (2017).
Tomioka, Y. et al. Decreased serum pyridoxal levels in schizophrenia: meta-analysis and Mendelian randomization analysis. J. Psychiatry Neurosci. 43, 194–200 (2018).
Misiak, B., Stramecki, F., Stanczykiewicz, B., Frydecka, D. & Lubeiro, A. Vascular endothelial growth factor in patients with schizophrenia: a systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 86, 24–29 (2018).
Goetz, R. L. & Miller, B. J. Meta-analysis of ghrelin alterations in schizophrenia: effects of olanzapine. Schizophrenia Res. 206, 21–26 (2019).
Fang, X., Zhang, Y., Fan, W., Tang, W. & Zhang, C. Interleukin-17 alteration in first-episode psychosis: a meta-analysis. Mol. Neuropsychiatry 3, 135–140 (2018).
Cao, B. et al. Leptin and adiponectin levels in major depressive disorder: a systematic review and meta-analysis. J. Affect. Disord. 238, 101–110 (2018).
Joe, P., Petrilli, M., Malaspina, D. & Weissman, J. Zinc in schizophrenia: a meta-analysis. Gen. Hosp. Psychiatry 53, 19–24 (2018).
Salagre, E. et al. Homocysteine as a peripheral biomarker in bipolar disorder: a meta-analysis. Eur. Psychiatry 43, 81–91 (2017).
Bender, A., Hagan, K. E. & Kingston, N. The association of folate and depression: a meta-analysis. J. Psychiatr. Res. 95, 9–18 (2017).
Song, J., Viggiano, A., Monda, M. & De Luca, V. Peripheral glutamate levels in schizophrenia: evidence from a meta-analysis. Neuropsychobiology 70, 133–141 (2014).
Guo, J., Liu, C., Wang, Y., Feng, B. & Zhang, X. Role of T helper lymphokines in the immune-inflammatory pathophysiology of schizophrenia: systematic review and meta-analysis. Nord. J. Psychiatry 69, 364–372 (2015).
Nishi, A. et al. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in schizophrenia. Schizophr. Bull. 40, 1154–1163 (2014).
Maia-de-Oliveira, J. P. et al. Nitric oxide plasma/serum levels in patients with schizophrenia: a systematic review and meta-analysis. Rev. Bras. Psiquiatr. (Sao Paulo, Braz.: 1999) 34(Suppl. 2), S149–S155 (2012).
Brouwer, A., Luykx, J. J., van Boxmeer, L., Bakker, S. C. & Kahn, R. S. NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies. Neurosci. Biobehav. Rev. 37, 1587–1596 (2013).
Valipour, G., Saneei, P. & Esmaillzadeh, A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 99, 3863–3872 (2014).
Bartoli, F., Crocamo, C., Clerici, M. & Carra, G. Second-generation antipsychotics and adiponectin levels in schizophrenia: a comparative meta-analysis. Eur. Neuropsychopharmacol. 25, 1767–1774 (2015).
Mazereeuw, G., Herrmann, N., Andreazza, A. C., Khan, M. M. & Lanctot, K. L. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr. Dis. Treat. 11, 2479–2491 (2015).
Carvalho, A. F. et al. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J. Psychiatr. Res. 59, 28–37 (2014).
Watson, S. et al. A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biol. Psychiatry 72, 943–949 (2012).
Soria, V. et al. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: a systematic review and narrative synthesis. Psychoneuroendocrinology 93, 8–19 (2018).
Sole, B. et al. Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int. J. Neuropsychopharmacol. 20, 670–680 (2017).
Fusar-Poli, P. et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull. 41, 892–899 (2015).
Sakuma, K. et al. Folic acid/methylfolate for the treatment of psychopathology in schizophrenia: a systematic review and meta-analysis. Psychopharmacology 235, 2303–2314 (2018).
Firth, J. et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 18, 308–324, https://doi.org/10.1002/wps.20672 (2019).
De La Rosa, M., Rutz, S., Dorninger, H. & Scheffold, A. Interleukin‐2 is essential for CD4+ CD25+ regulatory T cell function. Eur. J. Immunol. 34, 2480–2488 (2004).
Thornton, A. M., Donovan, E. E., Piccirillo, C. A. & Shevach, E. M. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+ CD25+ T cell suppressor function. J. Immunol. 172, 6519–6523 (2004).
Maes, M. & Carvalho, A. F. The Compensatory Immune-Regulatory Reflex System (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 55, 8885–8903 (2018).
Roomruangwong, C. et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol. Neurobiol. 57, 778–797, https://doi.org/10.1007/s12035-019-01737-z (2020).
Masdeu, J. C., Dalmau, J. & Berman, K. F. NMDA receptor internalization by autoantibodies: a reversible mechanism underlying psychosis? Trends Neurosci. 39, 300–310 (2016).
Leon-Caballero, J. et al. Bipolar disorder and antibodies against the N-methyl-d-aspartate receptor: a gate to the involvement of autoimmunity in the pathophysiology of bipolar illness. Neurosci. Biobehav. Rev. 55, 403–412 (2015).
Nestler, E. J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002).
Diniz, B. S. & Teixeira, A. L. Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. Neuromolecular Med. 13, 217–222 (2011).
Mainardi, M., Fusco, S. & Grassi, C. Modulation of hippocampal neural plasticity by glucose-related signaling. Neural Plast. 2015, 657928 (2015).
Park, S. E., Dantzer, R., Kelley, K. W. & McCusker, R. H. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 8, 12 (2011).
Duman, C. H. et al. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 198, 366–371 (2009).
Hossain, M. M., Mukheem, A. & Kamarul, T. The prevention and treatment of hypoadiponectinemia-associated human diseases by up-regulation of plasma adiponectin. Life Sci. 135, 55–67 (2015).
Solmi, M. et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther. Clin. Risk Manag. 13, 757–777 (2017).
Kalkman, H. O. & Feuerbach, D. Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol. Ther. 163, 82–93 (2016).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Thompson, P. M. et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182 (2014).
Stokes, C. S. et al. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin. Nutr. 35, 950–957 (2016).
Feczko, E. et al. The heterogeneity problem: approaches to identify psychiatric subtypes. Trends Cogn. Sci. 23, 584–601 (2019).
Andreazza, A. C. et al. Guidelines for the standardized collection of blood-based biomarkers in psychiatry: steps for laboratory validity—a consensus of the Biomarkers Task Force from the WFSBP. World J. Biol. Psychiatry 20, 340–351 (2019).
Beijers, L., Wardenaar, K. J., van Loo, H. M. & Schoevers, R. A. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol. Psychiatry 24, 888–900 (2019).
Bartoli, F., Lax, A., Crocamo, C., Clerici, M. & Carra, G. Plasma adiponectin levels in schizophrenia and role of second-generation antipsychotics: a meta-analysis. Psychoneuroendocrinology 56, 179–189 (2015).
Fu, S. P. et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediators Inflamm. 2014, 983401 (2014).
Girshkin, L., Matheson, S. L., Shepherd, A. M. & Green, M. J. Morning cortisol levels in schizophrenia and bipolar disorder: a meta-analysis. Psychoneuroendocrinology 49, 187–206 (2014).
Acknowledgements
Olesya Ajnakina is funded by the National Institute for Health Research (NIHR) (NIHR Post-Doctoral Fellowship—PDF-2018-11-ST2-020). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. A.R.B. is supported by productivity grants from the National Council for Scientific and Technological Development (CNPQ-1B) and the Program of Academic Productivity (PIPA) of the University of São Paulo Medical School. M.I.H. has received grants from the Pakistan Institute of Living and Learning (PILL), the Physician’s Services Incorporated (PSI) Foundation and the Stanley Medical Research Institute (SMRI). J.F. is supported by a Blackmores Institute Fellowship. M.B. has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation. M.B. is supported by a NHMRC Senior Principal Research Fellowship 1059660 and 1156072. K.L.L. has grants from the Alzheimer’s Association (PTC-18-543823), National Institutes of Health (R01AG046543), Canadian Institutes for Health Research (MOP 201803PJ8), Alzheimer’s Drug Discovery Foundation (grant #1012358) Alzheimer Society of Canada (Grant 15-17). E.V. has received grants from the Brain and Behaviour Foundation, the Generalitat de Catalunya (PERIS), the Spanish Ministry of Science, Innovation and Universities (CIBERSAM), EU Horizon 2020, and the Stanley Medical Research Institute. D.A.P. was partially supported by R37MH068376 from the National Institute of Mental Health and a NARSAD Distinguished Investigator Award, Brain & Behavior Research Foundation (grant #26950). N.H. has received research support from the Canadian Institute of Health Research, National Institute on Aging, Alzheimer Society of Canada, Alzheimer’s Association US, and Alzheimer’s Drug Discovery Foundation.
Author information
Authors and Affiliations
Contributions
A.F.C., M. Solmi, M. Sanches, M.O.M., K.L.L., and N.H. designed the study. A.F.C., M. Solmi, M.O.M., O.A., C.S., Y.R.S., C.S.L., G.P., Beatrice Bortolato, and Muhammad I. Husain screened and extracted the data. A.F.C., M. Solmi, M. Sanches, and M.O.M. analyzed the data. All authors contributed to the interpretation of the findings and provided meaningful intellectual contributions to the manuscript. The final version was read and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
A.F.C., Marco Solmi, M. Sanches, M.O.M., B.S., O.A., C.S., J.S., C.S.L., A.R.B., G.P., B.S.F., B.B., M.I.H., E.D., J.F., T.D.C., M.M., L.S., P.F.-P., P.A.K., M.F., J.R., and N.H. have no conflicts of interest to declare. M.B. has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier. K.L.L. has received consulting fees from AbbVie, Lundbeck/Otsuka, Pfizer, ICG Pharma, and Kondor in the last 3 years. E.V. has served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Abbott, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda. Over the past 3 years, D.A.P. has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass, Posit Science, and Takeda Pharmaceuticals and an honorarium from Alkermes for activities unrelated to the current work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carvalho, A.F., Solmi, M., Sanches, M. et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry 10, 152 (2020). https://doi.org/10.1038/s41398-020-0835-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-0835-5
This article is cited by
-
Genetic evidence for causal effects of immune dysfunction in psychiatric disorders: where are we?
Translational Psychiatry (2024)
-
Allocation of Users of Mental Health Services to Needs-Based Care Clusters: An Italian Pilot Study
Community Mental Health Journal (2024)
-
EEG-based spatio-temporal relation signatures for the diagnosis of depression and schizophrenia
Scientific Reports (2023)
-
Towards a youth mental health paradigm: a perspective and roadmap
Molecular Psychiatry (2023)
-
Evidence of genetic overlap and causal relationships between blood-based biochemical traits and human cortical anatomy
Translational Psychiatry (2022)