Abstract
Background
To evaluate sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively) of renal ultrasonography (US) in predicting renal uptake defects or reduced renal function at Tc-99m dimercaptosuccinic acid (DMSA) scan (primary outcome). We also evaluated which factors could be associated with Tc-99m DMSA renal scan anomalies.
Methods
We retrospectively included all the patients with vesico-ureteral reflux (VUR) undergoing the first Tc-99m DMSA renal scan within 3 months from the most recent renal US between 2016 and 2018.
Results
Sensitivity, specificity, PPV, and NPV of US in predicting abnormal Tc-99m DMSA scan were 38.9%, 91.5%, 71.9%, and 72.9%, respectively. Different length between the kidneys, expressed as standard deviation score (SDS), showed an area under the receiver operating characteristic curve of 0.70 (95% CI, 0.60–0.80; p < 0.0001) when evaluated as predictor of abnormal Tc-99m DMSA scan. A different length between the two kidneys >1.11 SDS had 91.5% sensitivity and 57.6% specificity. At multivariate analysis, the factors with significantly increased odds ratio of abnormal Tc-99m DMSA scan were difference in length between two kidneys >1.11 SDS and dilated VUR.
Conclusions
The Tc-99m DMSA scan remains the gold standard to detect renal parenchymal anomalies. A different length between the kidneys >1.11 SDS and dilated VUR are predictors of abnormal Tc-99m DMSA renal scan.
Similar content being viewed by others
Introduction
In the past, urinary tract infections (UTIs) were considered as major determinants of vesico-ureteral reflux (VUR) nephropathy.1 Despite febrile UTIs can determine renal scars in predisposed subjects and in the presence of specific conditions,2,3 it has been shown that the VUR nephropathy could be determined by a congenital renal dysplasia associated with VUR.4,5 At the present time, the Tc-99m dimercaptosuccinic acid (DMSA) renal scan represents the diagnostic gold standard for parenchymal uptake defects.6
In the past decades, the Tc-99m DMSA renal scan was a constant exam in the diagnostic imaging procedures of a child having presented a febrile UTI.7 Moreover, the top–down approach recommended Tc-99m DMSA renal scan during the first febrile UTI and a late Tc-99m DMSA renal scan if the first was positive.8 However, the current guidelines for the management of a first febrile UTI in children have been modified, favoring less aggressive imaging strategies. The American Academy of Pediatrics does not recommend Tc-99m DMSA renal scan,9 while National Institute for Clinical Excellence10 and Italian society of Pediatric Nephrology11 suggest it 4 months10 or 6 months11 following UTI if atypical UTI or if VUR grade IV/V was observed, respectively.
Since Tc-99m DMSA renal scan exposes children to a non-negligible dose of ionizing radiations,12 it could be useful in the clinical practice to identify predictive factors of renal anomalies at Tc-99m DMSA scan to further optimize the execution of this technique with a radiation-sparing approach. Therefore, we designed a retrospective diagnostic test accuracy study in which the reference standard was the Tc-99m DMSA scan and the test was the renal ultrasonography (US). The aims of our study were to evaluate sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively) of renal US in predicting renal uptake defects or reduced renal function at Tc-99m DMSA scan (primary outcome) in overall population and in patients having shown only one febrile UTI, recurrent febrile UTIs, and no UTIs before the VUR diagnosis. We also evaluated which factors could be associated with Tc-99m DMSA renal scan anomalies (secondary outcomes).
Patients and methods
Inclusion/exclusion criteria
We retrospectively included all the patients with VUR undergoing the first Tc-99m DMSA renal scan between April 2016 and April 2018. Inclusion criteria were: (i) Tc-99m DMSA renal scan made within 3 months from the most recent renal US; (ii) Tc-99m DMSA renal scan made at least 4 months after the last UTI in patients who had presented UTI.10,13 The study was approved by our Research Ethical Committee. Informed consent was obtained before any procedure.
Exclusion criteria were: (i) congenital solitary functioning kidney; (ii) suspect of obstructive uropathies (posterior urethral valve, megaureter (>10 mm), and pyelectasis (>15 mm)); (iii) acute events between US and Tc-99m DMSA renal scan; (iv) denied consent.
VUR diagnosis
As usually made in the clinical practice of our center, VUR had been diagnosed with cystography in males and with cystoscintigraphy in females. For the patients who had presented a febrile UTI, we followed the recommendations of the Italian Society of Pediatric Nephrology when screening which patients to submit cystography/cystoscintigraphy.14 Otherwise, we selected the patients to submit cystography/cystoscintigraphy on the basis of US risk factor for VUR.15
Clustering of VUR grades was adapted, classifying Grades III–V as “dilated” and Grades I–II as “non-dilated.”16
UTI definition
Febrile UTIs were defined by the presence of urinary leukocytes and/or nitrites, positive urine culture, and fever >38 °C. Urine culture was positive in the presence of ≥105 colonies/mL of a single species on a sample obtained by midstream clean catch specimens or sterile bag after cleaning of genital area17 or >104 colonies/mL on samples obtained by bladder catheterization. The bladder catheterization was always employed in febrile non-toilet-trained patients.
On the basis of UTIs, patients were divided into three groups: patients having shown only one UTI (Group 1), recurrent UTIs (Group 2), and no UTIs (Group 3) before the VUR diagnosis.
Definition of abnormal renal US
Renal US has been made by the same group of three pediatric nephrologists with specific abilities in renal US. The standard deviation score (SDS) of renal length was calculated as previously described.18 The SDS of renal length was calculated when the renal US was made. When we analyzed that retrospective data, we obtained the difference in SDS between the length of the two kidneys of each patient as follows: SDS of the longer kidney − SDS of the shorter kidney.
Abnormal US was defined by renal length SDS <2 (defined as “small kidney” in this manuscript), cortical defects (scars detected at US), diminished corticomedullary differentiation, and hyperechoic renal cortex. Cortical defects, diminished corticomedullary differentiation, and hyperechoic renal cortex were clustered as “US-detected renal parenchymal anomalies” for the analyses in this manuscript.
Definition of abnormal Tc-99m DMSA renal scan
The Tc-99m DMSA scan has always been made by the same operator and in blind about the results of the renal US. Abnormal Tc-99m DMSA scan was defined by relative renal function <45% and/or cortical uptake defects.19
Statistical analysis
p Values <0.05 were considered statistically significant. Differences for continuous variables were analyzed with the independent-sample t test for normally distributed variables and with Mann–Whitney test in case of non-normality. Qualitative variables were compared by using X2 test. We calculated sensitivity, specificity, PPV, and NPV of different parameters in predicting renal uptake defects or reduced renal function at Tc-99m DMSA scan. The difference in length between the two kidneys, expressed as SDS, was evaluated as a potential predictor of abnormal Tc-99m DMSA renal scan by receiver-operating characteristic (ROC) curves analysis. We also made a ROC curve analysis for dilated VUR, difference in length between two kidneys >1.11 SDS, and combination of both of them, expressed as binary variables (yes/no).
Logistic regression models were used to perform a univariate and multivariate analysis of predictors of abnormal Tc-99m DMSA renal scan. To explore associations with abnormal Tc-99m DMSA renal scan, a univariate analysis was performed for the following variables: age at VUR diagnosis (linear), gender (male/female), UTIs (yes/no), recurrent UTIs (yes/no), dilated VUR (yes/no), abnormal US (yes/no), small kidney (yes/no), US-detected renal parenchymal anomalies (yes/no), and different length between the two kidneys >1.11 SDS (yes/no). We decided to include in the multivariate analysis all the possible variables with a p ≤ 0.10 at univariate analysis and age and gender. The Stat-Graph XVII software for Windows was used for all statistical analyses except for the ROC curve analysis that was performed with Graphpad Prism 7.
Results
Patients’ characteristics
In the study period, we examined 298 eligible patients. Seventy-seven patients were excluded because of a suspect of obstructive uropathies, 40 for congenital solitary kidney, 12 for denied consent to Tc-99m DMSA renal scan execution, and 4 for an acute event between US and Tc-99m DMSA renal scan. Therefore 165 patients were included.
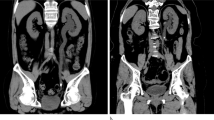
The patients’ characteristics are described in the Table 1. Focusing on the radiological anomalies, abnormal renal US was found in 32 patients while abnormal Tc-99m DMSA scan was found in 59 patients (Fig. 1).
Radiological anomalies. a Renal ultrasonographic anomalies. b Tc-99m DMSA renal scan anomalies. We found uptake defects (scars) in 43 patients (17 with only uptake defects and 26 with uptake defects and reduced relative function). The interval time between the last UTI and Tc99m DMSA renal scan in these patients was ≥6 months in 23 patients, ≥5 months and <6 months in 13 patients, and ≥4 months and <5 months in 7 patients.
Tc-99m DMSA renal scan resulted abnormal in 6 out of 11 patients (54.5%) with isolated US-detected renal parenchymal anomalies, in 8 out of 9 patients (88.9%) with US-detected renal parenchymal anomalies and small kidney, and in 9 out of 12 patients (75%) with isolated small kidney (p = 0.22).
Primary outcome
Table 2 shows sensitivity, specificity, PPV, and NPV of renal US in predicting abnormal Tc-99m DMSA scan in overall population and in patients having shown only one UTI (Group 1), recurrent UTIs (Group 2), and no UTIs (Group 3) before the VUR diagnosis.
The difference in length between the two kidneys, expressed as SDS, was evaluated as potential predictor of abnormal Tc-99m DMSA renal scan with the ROC curve analysis. The area under the ROC curve was 0.70 (95% confidence interval (CI), 0.60–0.80; p < 0.0001). A different length between the two kidneys >1.11 SDS had 91.5% (95% CI 84.5–96.1%) sensitivity and 57.6% (95% CI 44.1–70.4%) specificity for predicting abnormal Tc-99m DMSA renal scan (Fig. 2).
Secondary outcomes
Table 3 shows sensitivity, specificity, PPV, NPV, and area under ROC curve for detection of abnormal Tc-99m DMSA renal scan of dilated VUR; difference in length between two kidneys >1.11 SDS; and combination of both of them. Dilated VUR had the best sensitivity and different length between kidneys >1.11 SDS had the best NPV and highest area under the ROC curve, while combination of them had the best specificity and PPV.
At univariate analysis, male gender, abnormal US, US-detected renal parenchymal anomalies, small kidney, and the presence of dilated VUR showed a significantly increased odds ratio (OR) to predict abnormal Tc-99m DMSA renal scan (Fig. 3a). At multivariate analysis, the only factors with a significantly increased OR were difference in length between two kidneys >1.11 SDS and dilated VUR (model R2 39.4%, p < 0.0001) (Fig. 3b). The simultaneous presence of difference in length between two kidneys >1.11SDS and dilated VUR increased the OR to predict abnormal Tc-99m DMSA renal scan (OR = 13.1, 95% CI 4.2–40.7; p < 0.0001).
Discussion
The renal US is an easily accessible imaging technique but it is operator dependent. There are discordant literature data about renal US sensitivity in detecting renal parenchymal anomalies, making the role of renal US controversial.6,20,21,22,23,24,25,26,27 These differences could be explained by different criteria for interpretation of both renal US and Tc-99m DMSA renal scan. In our study, although the renal US had always been performed by the same group of three experienced operators in renal US and the Tc-99m DMSA renal scan had been made by only one radiologist, the sensitivity of renal US was low with excellent specificity (Table 2), as shown in several studies.6,20,21,22,26
At the present time, therefore, the Tc-99m DMSA renal scan remains the gold standard technique for the diagnosis of renal parenchymal defects.
To the best of our knowledge, until now, studies evaluating the impact of clinical and US factors in predicting anomalies at Tc-99m DMSA renal scan are lacking. We showed that dilated VUR had the best sensitivity for prediction of abnormal Tc-99m DMSA renal scan and that the combination of dilated VUR and different length between kidney >1.11 SDS had the best specificity. Different length between kidneys >1.11 SDS had the highest area under the ROC curve and this indicated that the ultrasonographic measurement of renal length could be the best test for prediction of abnormal Tc-99m DMSA renal scan. Moreover, at logistic regression analysis we found that the best predictors for abnormal Tc-99m DMSA renal scan were different length between the kidneys >1.11 SDS (OR 8.8, 95% CI 3.3–25.5) and dilated VUR (OR 2.7, 95% CI 1.2–6.38). The combination of them showed the highest OR (13.1; 95% CI 4.2–40). These findings support the recommendation of the Italian Society of Pediatric Nephrology to submit to Tc-99m DMSA renal scan patients with VUR grade IV/V11 or abnormal renal US.14
When submitting a patient to a diagnostic protocol, the benefits should be carefully considered. The current guidelines have shown different abilities in detecting renal scars with the most aggressive protocol showing high sensitivity for detecting abnormalities, which in some cases could be of questionable benefit to the infants, and it is burdened with high financial and radiation costs.28
The effect of cumulative radiation doses in children on risk of cancer should be considered.29 Therefore, we would suggest a “radiation-sparing personalized approach” limiting the use of Tc-99m DMSA renal scan to selected cases in which benefits could exceed costs (biological and economical). The clinical impact of multiple scars or nephropathy associated with VUR is represented by reduced relative function of the kidney with subsequent possible development of proteinuria or hypertension.30 Our findings could be useful in selecting—without further radiation exposure—patients at higher risk of reduced relative function of the kidney (dilated VUR and different length between the kidneys >1.11 SDS) and then patients who could benefit of a close clinical follow-up in order to diagnose complications in earlier time period. During the follow-up, these patients could undergo to renal scintigraphy only in view of a possible unilateral nephrectomy (i.e., for a hypertension related to poor function of a kidney or for recurrent UTIs associated with a poor functioning kidney with VUR).
Our study is limited by the retrospective design even if it has the advantage that these results could be easily reproducible in the daily clinical practice. In fact, the time dedicated to each scan was that of the “normal working day,” while in prospective studies a “special attention” could be paid. Moreover, although on one hand our sample size may have underpowered the analysis, on the other hand our cohort (165 patients with 330 renal units) is one of the largest available in literature. Another limitation could be represented by an overestimation of renal scars detected at Tc-99m DMSA scan for the 20 patients having done this exam with an interval <6 months since the last UTI and having shown renal uptake defects (Fig. 1b). In fact, it has been shown that a duration of 6 months could be needed to allow acute reversible lesions to disappear.31
In conclusion, our findings confirm that Tc-99m DMSA renal scan remains the gold standard technique for the detection of renal parenchymal anomalies and add to the current knowledge that different length between the kidneys >1.11 SDS and dilated VUR are the best predictors of abnormal Tc-99m DMSA renal scan. This information could be useful in the cost–benefit analysis when deciding if submitting a patient to Tc-99m DMSA renal scan.
References
Bailey, R. R. The relationship of vesico-ureteric reflux to urinary tract infection and chronic pyelonephritis-reflux nephropathy. Clin. Nephrol. 1, 132–141 (1973).
Shaikh, N. et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 170, 848 (2016).
Karavanaki, K. et al. Fever duration during treated urinary tract infections and development of permanent renal lesions. Arch. Dis. Child. 104, 466–470 (2019).
Ardissino, G. et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics 111, e382–e387 (2003).
Marzuillo, P. et al. Outcomes of a cohort of prenatally diagnosed and early enrolled patients with congenital solitary functioning kidney. J. Urol. 198, 1153–1158 (2017).
Marceau-Grimard, M. et al. Dimercaptosuccinic acid scintigraphy vs. ultrasound for renal parenchymal defects in children. Can. Urol. Assoc. J. 11, 260–264 (2017).
Kass, E. J., Kernen, K. M. & Carey, J. M. Paediatric urinary tract infection and the necessity of complete urological imaging. BJU Int. 86, 94–96 (2000).
Preda, I., Jodal, U., Sixt, R., Stokland, E. & Hansson, S. Normal dimercaptosuccinic acid scintigraphy makes voiding cystourethrography unnecessary after urinary tract infection. J. Pediatr. 151, 581–584 (2007).
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management& Roberts, K. B. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128, 595–610 (2011).
National Institute for Health and Clinical Excellence. Urinary tract infection in children: diagnosis, treatment and long-term management https://www.ncbi.nlm.nih.gov/pubmed/21290637 (2007).
Ammenti, A., et al. Updated Italian recommendations for the diagnosis, treatment and follow up of the first febrile urinary tract infection in young children. Acta Paediatr. https://doi.org/10.1111/apa.14988 (2019).
Fahey, F. H. et al. Dose estimation in pediatric nuclear medicine. Semin. Nucl. Med. 47, 118–125 (2017).
Expert Panel on Pediatric Imaging et al. ACR Appropriateness Criteria® Urinary Tract Infection-Child. J. Am. Coll. Radiol. 14, S362–S371 (2017).
Ammenti, A. et al. Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr. 101, 451–457 (2012).
Gordon, Z. N. et al. Uroepithelial thickening improves detection of vesicoureteral reflux in infants with prenatal hydronephrosis. J. Pediatr. Urol. 12, 257.e1-7 (2016).
Marzuillo, P. et al. Antibiotics for urethral catheterization in children undergoing cystography: retrospective evaluation of a single-center cohort of pediatric non-toilet-trained patients. Eur. J. Pediatr. 178, 423–425 (2019).
Marzuillo, P. et al. Cleaning the genitalia with plain water improves accuracy of urine dipstick in childhood. Eur. J. Pediatr. 177, 1573–1579 (2018).
Marzuillo, P. et al. Congenital solitary kidney size at birth could predict reduced eGFR levels later in life. J. Perinatol. 39, 129–134 (2018).
Patel, K., Charron, M., Hoberman, A., Brown, M. L. & Rogers, K. D. Intra- and interobserver variability in interpretation of DMSA scans using a set of standardized criteria. Pediatr. Radiol. 23, 506–509 (1993).
Moorthy, I., Wheat, D. & Gordon, I. Ultrasonography in the evaluation of renal scarring using DMSA scan as the gold standard. Pediatr. Nephrol. 19, 153–156 (2004).
Temiz, Y., Tarcan, T., Onol, F. F., Alpay, H. & Simşek, F. The efficacy of Tc99m dimercaptosuccinic acid (Tc-DMSA) scintigraphy and ultrasonography in detecting renal scars in children with primary vesicoureteral reflux (VUR). Int. Urol. Nephrol. 38, 149–152 (2006).
Veenboer, P. W. et al. Diagnostic accuracy of Tc-99m DMSA scintigraphy and renal ultrasonography for detecting renal scarring and relative function in patients with spinal dysraphism. Neurourol. Urodyn. 34, 513–518 (2015).
Roebuck, D. J., Howard, R. G. & Metreweli, C. How sensitive is ultrasound in the detection of renal scars? Br. J. Radiol. 72, 345–348 (1999).
Kersnik Levart, T. et al. Sensitivity of ultrasonography in detecting renal parenchymal defects in children. Pediatr. Nephrol. 17, 1059–1062 (2002).
Shanon, A. et al. Evaluation of renal scars by technetium-labeled dimercaptosuccinic acid scan, intravenous urography, and ultrasonography: a comparative study. J. Pediatr. 120, 399–403 (1992).
Sinha, M. D., Gibson, P., Kane, T. & Lewis, M. A. Accuracy of ultrasonic detection of renal scarring in different centres using DMSA as the gold standard. Nephrol. Dial. Transpl. 22, 2213–2216 (2007).
Rickwood, A. M. et al. Current imaging of childhood urinary infections: prospective survey. BMJ 304, 663–665 (1992).
La Scola, C. et al. Different guidelines for imaging after first UTI in febrile infants: yield, cost, and radiation. Pediatrics 131, e665–e671 (2013).
Kleinerman, R. A. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr. Radiol. 36, 121 (2006).
Peters, C. & Rushton, H. G. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J. Urol. 184, 265–273 (2010).
Stokland, E., Hellström, M., Jakobsson, B. & Sixt, R. Imaging of renal scarring. Acta Paediatr. Suppl. 88, 13–21 (1999).
Acknowledgements
The authors thank Simona Malvone for the revision of the written English.
Author contributions
Each author has met the Pediatric Research authorship requirements. Research idea and study design: P.M., S.G.; data acquisition: D.C., N.M., G.C., P.F.R.; data analysis/interpretation: P.M., A.L.M., E.M.D.G., S.G.; statistical analysis: P.M., E.M.D.G., S.G.; supervision or mentorship: N.M., G.C., P.F.R., A.L.M. Each author contributed important intellectual content during manuscript drafting or revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guarino, S., Capalbo, D., Martin, N. et al. In children with urinary tract infection reduced kidney length and vesicoureteric reflux predict abnormal DMSA scan. Pediatr Res 87, 779–784 (2020). https://doi.org/10.1038/s41390-019-0676-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0676-1
This article is cited by
-
Clinical implications of primary “occult” vesicoureteral reflux in male children
European Radiology (2024)
-
Body surface area-based kidney length percentiles misdiagnose small kidneys in children with overweight/obesity
Pediatric Nephrology (2023)
-
Evolution of congenital anomalies of urinary tract in children with and without solitary kidney
Pediatric Research (2022)
-
Demographic, clinical, and laboratory factors associated with renal parenchymal injury in Iranian children with acute pyelonephritis
BMC Infectious Diseases (2021)