Abstract
Background:
We hypothesized that acute kidney injury (AKI) in asphyxiated neonates treated with therapeutic hypothermia would be associated with hypoxic–ischemic lesions on brain magnetic resonance imaging (MRI).
Methods:
Medical records of 88 cooled neonates who had had brain MRI were reviewed. All neonates had serum creatinine assessed before the start of cooling; at 24, 48, and 72 h through cooling; and then on day 5 or 7 of life. A neonatal modification of the Kidney Disease: Improving Global Outcomes guidelines was used to classify AKI. MRI images were evaluated by a neuroradiologist masked to outcomes. Outcome of interest was abnormal brain MRI at 7–10 d of life.
Results:
AKI was found in 34 (39%) of 88 neonates, with 15, 7, and 12 fulfilling criteria for stages 1, 2, and 3, respectively. Brain MRI abnormalities related to hypoxia–ischemia were present in 50 (59%) newborns. Abnormal MRI was more frequent in infants from the AKI group (AKI: 25 of 34, 73% vs. no AKI: 25 of 54, 46%; P = 0.012; odds ratio (OR) = 3.2; 95% confidence interval (CI) = 1.3–8.2). Multivariate analysis identified AKI (OR = 2.9; 95% CI = 1.1–7.6) to be independently associated with the primary outcome.
Conclusion:
AKI is independently associated with the presence of hypoxic–ischemic lesions on postcooling brain MRI.
Similar content being viewed by others
Main
Acute kidney injury (AKI) is common in neonates suffering from perinatal asphyxia (1,2,3,4,5,6). Before the advent of therapeutic hypothermia, studies evaluating the relationship of renal and central nervous system injury in asphyxiated newborns reported acute renal failure to be significantly associated with worsening hypoxic–ischemic encephalopathy (HIE), and adverse long-term neurodevelopmental outcome (5,7,8). Previous studies describing AKI in asphyxiated newborns have relied on differing definitions of AKI, making comparisons between studies difficult. Jetton and Askenazi (9) have proposed a modification of the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition of AKI as a standardized definition of AKI for neonates, which is based on the rise in serum creatinine (SCr) from a previously documented low, rather than absolute, SCr thresholds. This and similar definitions have gained acceptance as a way to define AKI in newborns (9,10). A similar definition was used to study AKI in neonates with perinatal asphyxia not receiving therapeutic hypothermia (6) and recently by our group in neonates treated with therapeutic hypothermia for perinatal asphyxia (11). To date, the correlation between AKI and severity of hypoxic–ischemic injury on brain magnetic resonance imaging (MRI) has not been evaluated in neonates treated with therapeutic hypothermia.
Therapeutic hypothermia, which has become the standard of care for asphyxiated newborns, improves the hypoxic–ischemic lesion on brain MRI (an important proxy predictor for long-term neurodevelopmental outcomes) (12), and presumably also may ameliorate AKI (13,14). Growing evidence in animal models suggest that AKI is not an isolated event but results in remote organ dysfunction involving the heart, lungs, liver, intestines, and brain through an inflammatory mechanism that involves neutrophil migration, cytokine expression, and increased oxidative stress (15). Thus, brain injury after perinatal asphyxia may be independently associated with AKI. We hypothesized that AKI would be associated with hypoxic–ischemic lesions on brain MRI and that the severity of lesions would parallel worsening AKI. The aim of the study was to determine whether AKI during therapeutic hypothermia predicts the presence of subsequent brain MRI abnormalities related to hypoxia–ischemia.
Results
Brain MRI scans were available for analysis in 88 of 96 cooled infants. Two neonates, who died before MRI could be obtained, and another six infants, who had other types of neuroimaging studies (cranial sonography or computed tomography) and not brain MRI, were excluded.
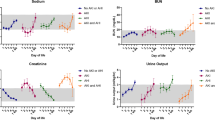
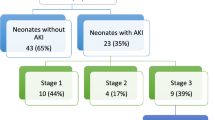
AKI was found in 34/88 (39%) neonates with 15, 7, and 12 fulfilling the criteria for stages 1, 2, and 3, respectively. The mean SCr was higher in the AKI group before the start of cooling and all through cooling ( Table 1 ).
Median postnatal age at brain MRI was 8 d (interquartile range: 7–9 d). Brain MRI abnormalities related to hypoxia–ischemia were present in 50/88 (59%) neonates. In 26 neonates (AKI: 14; no AKI: 12), brain MRI abnormality was “more extensive.”
Abnormal brain MRI was more frequent in neonates with AKI vs. neonates with no AKI (25/34 (73%) vs. 25/54 (46%); odds ratio (OR): 3.2; 95% confidence interval (CI): 1.3–8.2; P = 0.012). Other variables associated with abnormal MRI include receipt of chest compression during resuscitation (OR: 3.6; 95% CI: 1.5–8.8; P = 0.004), clinical seizure (OR: 2.5; 95% CI: 1.05–6.1; P = 0.036), and absence of spontaneous movement (OR: 2.4; 95% CI: 1.0–6; P = 0.047), as depicted in Table 2 . Stepwise logistic regression analysis of the significant variables with P value ≤0.2 from the univariate analysis (AKI, receipt of chest compression, clinical seizure, absence of spontaneous movement, male gender, presence of an intrapartum sentinel event, and continued need for ventilation for more than 10 min for resuscitation) identified only the AKI (P = 0.032; OR: 2.9; 95% CI: 1.1–7.6), and receipt of chest compression during resuscitation (P = 0.010; OR: 3.3; 95% CI: 1.3–8.3) to be independently associated with the primary outcome.
As AKI and the need for chest compressions both may trend with the severity of asphyxia, there can be significant interaction between AKI and the need for chest compressions, and it might lead one to overstate the significance of the two variables. Hence, we reanalyzed the data after adding AKI × chest compressions interaction variable to other significant variables with a P value ≤0.05 on univariate analysis (AKI, chest compression, seizure, and absence of spontaneous movement) in the logistic regression model, but the AKI × chest compressions interaction variable was not retained, indicating no effect on the multivariate model.
Table 3 shows the correlation of AKI stages and severity of brain MRI abnormality. No difference was found in the number of “more extensive” MRI abnormality between AKI and no-AKI groups [14/34 (41%) vs. 12/54 (22%); OR: 0.4; 95% CI: 0.16–1.04; P = 0.091). “More extensive” MRI abnormality in neonates with stage 3 AKI was not different compared with that in neonates with stage 2 (stage 3 AKI: 3 of 12 (25%) vs. stage 2 AKI: 3 of 7 (43%); P = 0.617; OR: 2.3; 95% CI: 0.3–16.4) or stage 1 AKI (stage 3 AKI: 3 of 12 (25%) vs. stage 1 AKI: 8 of 15 (53%); P = 0.238; OR: 3.4, 95% CI: 0.6–18; Table 4 ).
Discussion
The importance and prognostic significance of AKI in the neonatal period is becoming increasingly apparent (6,11,16,17). Using contemporary categorical SCr-based definitions, we report for the first time that AKI is associated with posthypothermia brain MRI abnormalities even after controlling for other important predictors of abnormal MRI in neonates treated with therapeutic hypothermia. Even after controlling for other risk factors, demographics, and interventions associated with perinatal asphyxia, those with AKI had 2.9 times higher odds of abnormal MRI than those without AKI.
Our findings extend those first reported by Perlman and Tack (8) examining the association of AKI with long-term neurological outcomes in asphyxiated newborns before therapeutic hypothermia becoming the standard of care. This original study used clinical markers of HIE and a definition of AKI dependent on oliguria. We extend these findings by showing that AKI is associated with imaging abnormalities at 7–10 d of life by using systematic definitions for AKI and brain MRI. However, it is possible that AKI, similar to the need for chest compression for resuscitation, is acting as a marker for the severity of asphyxia and that severer asphyxia is associated with abnormal brain MRI findings. Nevertheless, our findings provide the clinician with a marker (AKI) during the course of therapeutic hypothermia, which may help to stratify patients at risk for an adverse neurological outcome.
Although we demonstrate AKI to be predictive of an abnormal posthypothermia brain MRI, we noted that the severity of AKI during cooling did not correlate with the extent of brain MRI abnormalities. There are several potential explanations of these findings. One lies in the known pathophysiologic mechanisms of the perinatal response to fetal distress that shunts blood away from the kidneys to the brain and other vital organs. There indeed may be a heterogeneity in this response across individuals relative to the circumstances around birth that leads to the differences seen in the degree of AKI and the extent of MRI abnormalities. The similar incidence of “more extensive” MRI abnormality following hypothermia treatment in infants with different stages of AKI may also reflect differential beneficial effects of hypothermia on the brain and kidney. Although we believe that the AKI definition used here is the best definition of AKI currently available for asphyxiated infants receiving therapeutic hypothermia, the AKI-staging criteria may yet be imprecise with substantial severity overlap (especially between stages 2 and 3) and may poorly stratify outcomes. Future studies using novel biomarkers of kidney function that have been shown to be more precise in the neonatal period may allow for more precise definitions of AKI (18). Limitations of the descriptions of the MRI categories may also be another reason for the lack of correlation between AKI and the MRI findings. In addition, we acknowledge that our small sample size and lack of power may explain why a clear progressive association between AKI and MRI severity was not evident in our data.
The findings we present are consistent with previous reports in the literature. Our reported incidence of 41% of normal brain MRI in neonates with perinatal asphyxia treated with therapeutic hypothermia is consistent with previous reports in the sentinel neuroprotection studies (12). Postulated mechanisms for hypothermic neuroprotection are nonspecific and, in theory, the beneficial effect of hypothermia should extend beyond the brain to include other organs commonly affected during a hypoxic–ischemic insult, including kidneys. Interestingly, the 39% incidence of AKI in our cohort that underwent therapeutic hypothermia was similar to that reported by Kaur et al. (6), also using the Acute Kidney Injury Network criteria in asphyxiated infants who did not receive therapeutic hypothermia. There were some important differences between the definitions used in these studies. Categorization of moderate to severe asphyxia in this study was based on published criteria from the Cool Cap or National Institute of Child Health and Human Development cooling protocols (19,20), including Apgar score at 5 min, cord blood pH, and base deficit, in addition to clinical signs of moderate to severe HIE, which might have selected severer cases of asphyxia for hypothermia treatment. Infants in the latter study were categorized into moderate to severe asphyxia based on 1-min Apgar score alone, which may overestimate the infant’s severity of asphyxia (21,22). To answer the question regarding whether the beneficial effects of therapeutic hypothermia extend to include the kidneys, it would be of benefit to evaluate data from already-published randomized controlled trials, and future studies on therapeutic hypothermia should consider using contemporary definitions.
The limitations of this study include the fact that this is a single-center retrospective analysis. Although we were able to find statistically significant associations, our small sample size limited the precision of our estimates in subpopulations. The categorization of brain injury in this study as “more extensive” abnormality on posthypothermia brain MRI was according to previously published reports (23,24). It is worth noting that this study was not designed to look at the long-term neurodevelopmental outcome in infants with AKI, and caution should be used in the interpretation of our findings. Current literature suggests that “MRI in the neonatal period is qualified as a biomarker of the disease and treatment response and might be of use in neuroprotective studies.” (12) It is important to recognize that in the absence of long-term neurodevelopmental follow-up, it is not clear whether residual MRI abnormalities after therapeutic hypothermia in infants with or without AKI represent the final neuropathology or the injury that may still be evolving. Nevertheless, this study emphasizes the importance of recognizing AKI in asphyxiated newborns receiving therapeutic hypothermia. Our study also highlights the need for meta-analysis of individual patient data from the previous cooling trials to determine whether AKI using the modified Acute Kidney Injury Network criteria predicts long-term neurodevelopmental outcome for infants treated with hypothermia.
Methods
The medical records of 96 consecutive cases of asphyxiated newborns treated with therapeutic cooling according to the Cool Cap or National Institute of Child Health and Human Development cooling protocols between 2003 and 2010 were retrospectively reviewed (19,20). As per protocols, term and late-preterm infants of ≥36 wk’ gestation with HIE, who were ≤6 h of age and who had clinical evidence of exposure to perinatal hypoxia–ischemia (Apgar score <5 at 10 min of age, continued need for ventilation and resuscitation at 10 min of age, and metabolic acidosis with pH <7 or base deficit >16 mmol/l in cord or arterial blood gases measured within 1 h of birth), in addition to an abnormal neurological examination of moderate (Sarnat stage 2) or severe (Sarnat stage 3) encephalopathy or clinical seizure received therapeutic hypothermia (19,20). The Cool Cap protocol required an abnormal amplitude-integrated electroencephalography recording as an additional criterion (19). Infants with major congenital abnormality or severe growth restriction, and moribund infants for whom no further aggressive treatment was planned, were not cooled.
All these neonates had SCr and serum electrolytes assessed before the start of cooling (baseline), at specific time intervals (24, 48, and 72 h) through cooling, and then on day 5 or 7 of life. Neuroimaging studies, usually MRI of the brain, were performed in all cooled infants at around 7–10 d of life per unit protocol. Written informed consent was obtained from a parent before the start of cooling for all infants, and this retrospective review was approved by the Institutional Review Board at the University of Michigan.
Data collection included details of the prenatal or intrapartum events, including presence of meconium-stained amniotic fluid, mode of delivery, and clinically identifiable intrapartum sentinel event (placental abruption, cord prolapse, cord avulsion, vasa previa, ruptured uterus, maternal cardiopulmonary arrest, difficult delivery with shoulder dystocia); selected prehypothermia clinical and laboratory variables to assess the severity of asphyxia, including presence of asystole at birth, need for chest compression for resuscitation, Apgar scores of 0–3 at 5 min, a continued need for resuscitation with endotracheal or mask ventilation at 10 min after birth, and presence of severe acidosis defined as pH less than 7.00 or a base deficit of 16 mmol/l or more in an umbilical cord blood sample or an arterial or venous blood sample obtained within 60 min of birth; characteristics of the early neonatal course, including onset of clinical seizure activity before the start of hypothermia, abnormal early neurological examination (flaccidity, decerebrate posturing, and absence of spontaneous activity); and the subsequent neonatal course, including details of AKI if present, need for dialysis, and brain MRI findings.
Table 5 shows the SCr-based neonatal modification of the 2012 KDIGO criteria that was used to classify AKI (9,10,25). The modifications to the KDIGO criteria included the following: (i) the exclusion of urine output as AKI in neonates is often nonoliguric and (ii) a SCr cutoff of 2.5 mg/dl for stage 3, which reflects a comparable degree of renal failure with a SCr level of 4 mg/dl in adults (16).
The T1- and T2-weighted imaging sequences on the MRI scans were independently reviewed for the presence of abnormal signal intensities consistent with hypoxic–ischemic injury on brain MRI and categorized (for this study) by a neuroradiologist (J.R.B.) masked to the clinical history, original MRI report during initial admission, or outcome. There was complete agreement regarding the presence or absence of hypoxic–ischemic injury on brain MRI as reported by the neuroradiologists and pediatric neurologists during the initial admission and on subsequent reviews by the neuroradiologist coinvestigator (J.R.B.) for this study. The primary outcome was the presence of any hypoxic–ischemic lesions on posthypothermia brain MRI. Abnormal signal intensities consistent with hypoxic–ischemic injury to both basal nuclei (basal ganglia and thalamus) and cortex with or without extension beyond the watershed areas were considered “more extensive” brain MRI abnormalities. Hypoxic–ischemic lesions either in the basal nuclei (basal ganglia and thalamus) or in the cortex were considered “less extensive.” The categorization of the brain injury into “less extensive” and “more extensive” represent scores 1 or 2; and scores 3 or 4, respectively, of the basal ganglia/watershed scoring system described by Barkovich et al., and we have used this scoring system previously to assess the severity of posthypothermia brain MRI abnormalities (23,24,26,27).
Brain MRI is now recognized as a useful biomarker and potential surrogate end point in neuroprotection trials (28). The presence and the extent of brain MRI lesions was the selected outcome because information regarding long-term neurodevelopmental outcomes was not available for all infants.
Statistical Methods
SCr values were compared between the neonates with and without AKI. Normally distributed continuous variables were compared using Student’s t-test, whereas the categorical variables were tested using χ2 tests. Two-sided P values <0.05 were regarded as significant.
The relations among all of the selected prehypothermia clinical and laboratory variables, presence or absence of AKI, and the primary outcome of an abnormal brain MRI were assessed by univariate analysis. The selected prehypothermia covariates for this exploratory analysis, including pH, base deficit, and the specific components of the neurological examination to assess the severity of asphyxia, were based on previous reports, and they were similar to those previously identified to be predictive of severe disability or death in infants with HIE despite therapeutic hypothermia (29). Significant variables with a P value ≤0.2 from the univariate analysis were used in the stepwise forward logistic regression model to determine which of the variables were independently associated with the primary outcome of interest (abnormal brain MRI). In the forward logistic model, the P value for adding variables was set at 0.05 and for removing variables, the P value was set at 0.10. Data analysis was performed using commercially available statistical software (PASW 18, SPSS, Chicago, IL).
Statement of Financial Support
D.T.S. is supported by the Research Training in Pediatric Nephrology grant (T-32 F023015). D.J.A. is supported by the American Society of Nephrology Norman Siegel Career Development Award.
Disclosure:
D.J.A. is a consultant for Gambro Renal Products (Lakewood, CO).
References
Karlowicz MG, Adelman RD . Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol 1995;9:718–22.
Hankins GD, Koen S, Gei AF, Lopez SM, Van Hook JW, Anderson GD . Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol 2002;99(5 Pt 1):688–91.
Agras PI, Tarcan A, Baskin E, Cengiz N, Gürakan B, Saatci U . Acute renal failure in the neonatal period. Ren Fail 2004;26:305–9.
Aggarwal A, Kumar P, Chowdhary G, Majumdar S, Narang A . Evaluation of renal functions in asphyxiated newborns. J Trop Pediatr 2005;51:295–9.
Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP . Renal failure in asphyxiated neonates. Indian Pediatr 2005;42:928–34.
Kaur S, Jain S, Saha A, et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann Trop Paediatr 2011;31:129–34.
Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J . Multiple organ involvement in perinatal asphyxia. J Pediatr 1995;127:786–93.
Perlman JM, Tack ED . Renal injury in the asphyxiated newborn infant: relationship to neurologic outcome. J Pediatr 1988;113:875–9.
Jetton JG, Askenazi DJ . Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24:191–6.
Mehta RL, Kellum JA, Shah SV, et al.; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S . Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr 2013;162:725–729.e1.
Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45.
Róka A, Vásárhelyi B, Bodrogi E, Machay T, Szabó M . Changes in laboratory parameters indicating cell necrosis and organ dysfunction in asphyxiated neonates on moderate systemic hypothermia. Acta Paediatr 2007;96:1118–21.
Sarkar S, Barks JD, Bhagat I, Donn SM . Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol 2009;29:558–63.
Yap SC, Lee HT . Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 2012;116:1139–48.
Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D . Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res 2011;69:354–8.
Askenazi DJ, Ambalavanan N, Goldstein SL . Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol 2009;24:265–74.
Askenazi DJ, Koralkar R, Hundley HE, et al. Urine biomarkers predict acute kidney injury in newborns. J Pediatr 2012;161:270–5.e1.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Shankaran S, Laptook AR, Ehrenkranz RA, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84.
Ellis M, Manandhar N, Manandhar DS, deL Costello AM . An Apgar score of three or less at one minute is not diagnostic of birth asphyxia but is a useful screening test for neonatal encephalopathy. Indian Pediatr 1998;35:415–21.
Mercuri E, Rutherford M, Barnett A, et al. MRI lesions and infants with neonatal encephalopathy. Is the Apgar score predictive? Neuropediatrics 2002;33:150–6.
Sarkar S, Donn SM, Bapuraj JR, Bhagat I, Dechert RE, Barks JD . The relationship between clinically identifiable intrapartum sentinel events and short-term outcome after therapeutic hypothermia. J Pediatr 2011;159:726–30.
Sarkar S, Donn SM, Bapuraj JR, Bhagat I, Barks JD . Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: selective head versus whole body cooling. Arch Dis Child Fetal Neonatal Ed 2012;97:F335–9.
KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012;suppl 2:1–138.
Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–9.
Sarkar S, Barks JD, Bapuraj JR, et al. Does phenobarbital improve the effectiveness of therapeutic hypothermia in infants with hypoxic-ischemic encephalopathy? J Perinatol 2012;32:15–20.
Neil J . Is MRI still cool after hypothermia? Lancet Neurol 2010;9:19–20.
Ambalavanan N, Carlo WA, Shankaran S, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics 2006;118:2084–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, S., Askenazi, D., Jordan, B. et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res 75, 431–435 (2014). https://doi.org/10.1038/pr.2013.230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.230