Abstract
Tenascin-C (TNC), a large hexameric extracellular glycoprotein, is a pleiotropic molecule with multiple domains binding to a variety of receptors mediating a wide range of cellular functions. We earlier reported that TNC induces epithelial–mesenchymal transition (EMT)-like change in breast cancer cells. In the present study, we clarified TNC receptor involvement in this process. Among integrins previously reported as TNC receptors, substantial expression of αv, α2, β1 and β6 subunits was detected by quantitative PCR and immunoblotting in MCF-7 cells. Integrin β6 mRNA was remarkably upregulated by transforming growth factor (TGF)-β1 treatment, and protein expression was prominently increased by additional exposure to TNC. Immunofluorescent labeling demonstrated integrin αvβ6 accumulation in focal adhesions after TNC treatment, especially in combination with TGF-β1. The α2 and β1 subunits were mainly localized at cell–cell contacts, αv being found near cell cluster surfaces. Immunoprecipitation showed increase in αvβ1 heterodimers, but not α2β1, after TNC treatment. Activated β1 subunits detected by an antibody against the Ca2+-dependent epitope colocalized with αv in focal adhesion complexes, associated with FAK phosphorylation at tyrosine 925. Neutralizing antibodies against αv and β1 blocked EMT-like change caused by TNC alone. In addition, anti-αv and combined treatment with anti-β1 and anti-αvβ6 inhibited TGF-β1/TNC-induced EMT, whereas either of these alone did not. Integrin subunits αv, β1 and β6, but not α2, bound to TNC immobilized on agarose beads in a divalent cation-dependent manner. Treatments with neutralizing antibodies against β1 and αvβ6 reduced αv subunit bound to the beads. Immunohistochemistry of these receptors in human breast cancer tissues demonstrated frequent expression of β6 subunits in cancer cells forming scattered nests localized in TNC-rich stroma. These findings provide direct evidence that binding of αvβ6 and αvβ1 integrins to TNC as their essential ligand induces EMT-like change in breast cancer cells.
Similar content being viewed by others
Introduction
Tenascin-C (TNC) is an extracellular matrix glycoprotein formed by hexamers of 200–400 kDa subunits. Although expression of TNC is rare in normal adult tissues, high levels are observed in pathological states featuring tissue remodeling, such as inflammation, wound healing and cancer.1, 2, 3 In breast cancer tissues, TNC also demonstrates upregulated production by both tumor and stromal cells.4 Immunohistochemistry has shown TNC expression to be indicative of a poorer patient outcome in invasive ductal carcinoma cases.5 Low expression levels of miR-335, allowing high TNC expression, are linked to lung metastasis by human breast cancer.6 In a mouse model, TNC knockdown causes dramatic inhibition of lung metastasis and colonization by breast cancer cells.7 In vitro studies have shown that TNC promotes proliferation and migration of cancer cells8, 9, 10, 11 and increases their expression of matrix metalloproteinases.11, 12
Epithelial–mesenchymal transition (EMT) is an important event in cancer progression associated with invasion and metastasis.13, 14 A role for TNC in this process is also suggested by its frequent presence in invasive borders of cancer tissues and in microinvasive foci around intraductal components undergoing EMT.9, 15, 16, 17 Our recent study demonstrated TNC immunolabelling to be more frequently observed in stroma in the periphery of tumors featuring scattered cancer nests than in more solid lesions.18 We also reported that TNC addition to culture medium induces EMT-like change in human breast cancer MCF-7 cells, accompanied by SRC activation and focal adhesion kinase (FAK) phosphorylation.18 It has been reported that EMT induced by elevated SRC activity is blocked by neutralizing antibodies against αv or β1, suggesting a requirement for signaling through integrin adhesion complexes.19 A requirement for αv integrin subunits was also clarified in TNC-induced EMT,18 but the involvement of TNC receptors was not elucidated in detail. Interactions of cells with TNC may be mediated by members of the integrin family, heterodimers of α2/7/8/9β1 and αvβ1/3/6 as TNC receptor candidates.1, 2 In a colon cancer in vitro model, tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β1 were found to cause upregulation of the β6 subunit and consequent increase of αvβ6 heterodimers contributing to EMT.20 Transfection of β6 subunits induced EMT in oral squamous cell carcinoma (SCC) cells with enhanced TNC expression.21 Furthermore, EMT of oral SCC driven by co-stimulation of epidermal growth factor with TGF-β1 is associated with elevated expression of α7 and β1 subunits and extracellular matrix proteins including laminin 332 and TNC.22 Upregulated expression of αvβ3 is also observed in TGF-β-induced EMT in murine mammary epithelial cells.23 Moreover, colon cancer cells transfected with α9β1 display an EMT phenotype,24 and pancreatic cancer cells plated on collagen I show EMT-like change mediated through integrin α2β1.25
In the present study using MCF-7 cells, we therefore investigated the contributions of multiple integrin receptors, examining expression and alteration during EMT of integrin subunits forming TNC receptors by quantitative PCR (qPCR) and western blotting, in addition to localization by immunofluorescence as well as affinity binding to immobilized TNC and effects of neutralizing antibodies. Subunit expression in human breast cancer tissues was also explored by immunohistochemistry.
Results
Detection of integrin receptors for TNC expressed in MCF-7 cells
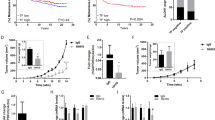
Integrins reported as TNC receptors include α2/7/8/9/v and β1/3/6 subunits.1, 2 Substantial mRNA expression of αv, α2, β1, and β6 subunits was validated by qPCR (Figure 1a), and these subunits were readily detected (Figure 2a) in immunoblot analysis of TGF-β1/TNC-treated MCF-7 cells. Compared with the Ct value for GAPDH, the values for integrin α7/8/9 and β3 subunits were extremely high, indicating insignificant expression in the treated cells. On immunoblotting, integrin β5 subunit was also detected as a positive control in this study, whereas integrin β3 was hardly present, in line with the qPCR findings.
Messenger RNA expression of TNC receptors of MCF-7 cells after TGF-β and TNC treatments. (a) mRNA was extracted from cells co-stimulated with TNC (10 μg/ml) and TGF-β1 (5 ng/ml) for synthesis of cDNA and performance of real-time PCR determining the cycle threshold (Ct) values for each subunit. Differences in Ct values of TNC receptor mRNAs from that of GAPDH mRNA in TGF-β1/TNC-treated cells are shown using qPCR. Ct values of α7/8/9 and β3 integrin subunits were very high, indicating only limited expression. Calibration curves were obtained using each target gene in the samples. (b) Changes of receptor mRNA expression for αv/2 and β1/6 after treatment with TGF-β1 and/or TNC. The results were normalized to GAPDH expression. Integrin αv showed significant increase after TGF-β1/TNC treatment and β6 was dramatically increased after TGF-β1 addition, with or without TNC (mean±s.d.; *P<0.05, ***P<0.001).
Immunoblot analyses of TNC receptor proteins of MCF-7 cells after TGF-β and TNC treatments. (a) Results of immunoblotting of integrin subunits of TNC receptors in MCF-7 cell lysates. (b) Quantification of bands of the subunit proteins in the treated cells showed significant increase in integrin αv after TNC- and TGF-β1/TNC treatments, as compared with the control case (Cont). While TNC addition significantly increased β6 amounts, TGF-β1/TNC did so more dramatically. The integrin α2 subunit showed slight but significant increase after TGF-β1 treatment (mean±s.d.; *P<0.05, **P<0.01, ***P<0.001). As a control, the β5 was not altered by any of the treatments.
TNC and TGF-β1 treatments increase αv and β6 expression
A significant increase of αv mRNA is observed with the combined treatment, whereas TGF-β1 treatment also showed a similar tendency but without statistical significance (Figure 1b). Expression of β6 mRNA after TGF-β1 and combined TGF-β1/TNC treatments was significantly increased to >10 times the control value, whereas expression of α2 and β1 was not altered. Immunoblotting demonstrated that protein amounts of αv subunits were significantly elevated by TNC and TGF-β1/TNC treatments (Figure 2b). The β6 amount was also significantly increased after TNC treatment and more prominently with TGF-β1/TNC, whereas it was lower in TGF-β1 treated cells, but not significantly. Elevated expression of β6 protein after the combined treatments was also noted in T-47D breast cancer cells (Supplementary Figure 1). The amount of α2 was slightly but significantly increased in TGF-β1- and TGF-β1/TNC-treated cells. The β1 amount was not different among the treatments. Although TGF-β1 upregulated mRNA expression of αv and β6 subunits, increases of the protein amounts were not significant. In contrast, TNC treatment did not evidently affect the mRNA expression, but contents of both proteins were significantly increased. TGF-β1/TNC-treated cells showed marked upregulation at both mRNA and protein levels. TNC addition with or without TGF-β1, but not TGF-β1 alone, to the medium induces EMT-like change of MCF-7 cells, this phenotype change being previously characterized in detail.18
Integrin αvβ6 heterodimers are recruited to focal adhesions by TNC treatment
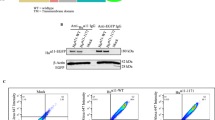
Immunofluorescence of αv subunits in control cells showed patchy staining of bottom surfaces of cell clusters with tiny focal adhesions at the cell peripheries. After TNC and TGF-β1/TNC treatments, the staining was intensely positive in larger adhesion plaques of spreading cells. Immunofluorescence of β6 demonstrated bright fluorescence in adhesion plaques of TNC- and TGF-β1/TNC-treated cells but not in control cells (Figure 3a) or cells treated with TGF-β1 alone (data not shown). Focal adhesions positive for β6 were observed more frequently in TGF-β1/TNC-treated cells than in TNC-treated ones, and colocalized in a part with αv-positive plaques (Figure 3b, arrows). Integrin β5 was observed in focal adhesions in accordance with integrin αv in all treated cells (Figure 3a). Plaques positive for β3 were barely detectable. Immunoprecipitation showed increasing β6 contents of integrin heterodimers co-precipitating with the αv subunit in TNC-treated cells and, more remarkably, in TGF-β1/TNC-treated ones (Figure 3c). Upregulated β6 protein may be recruited to focal adhesions as αvβ6.
Integrin β6 subunits are recruited to αv-positive adhesion plaques. (a) Immunofluorescence showed control cells to express αv (P2W7) and β5 integrin subunits, but not the β6 subunit. After TNC only and the TGF-β1/TNC treatment, β6-positive adhesion plaques were observed, more frequently in TGF-β1/TNC-treated cells. (b) Double immunofluorescence staining demonstrating colocalization of αv (Q-20) and β6 subunits. Scale bars: 20 μm. (c) Immunoprecipitation with anti-αv antibody (P2W7) showing prominent increase of co-precipitated β6 subunit in TGF-β1/TNC-treated cells. SDS-gel electrophoresis was performed under non-reducing conditions. As a negative control, a monoclonal antibody against a viral protein was used instead of anti-integrin antibodies. Whole-cell lysates (Lysate) of TGF-β1/TNC-treated cells were also examined.
Integrin α2β1 heterodimers are dominant in cell–cell contacts in control cells
Immunofluorescent staining of α2 and β1 demonstrated dominant localization at cell membranes and intercellular adhesions in untreated cells (Figure 4a). After treatment of TNC and TGF-β1/TNC, the membrane staining was diminished, coinciding with EMT-like change (Figure 4b). Immunofluorescence of α2 was not visible in focal adhesions. In control cells, αv subunits appeared localized on surfaces of cell clusters and cell–cell contacts near the surfaces (Figure 4a). Immunoprecipitation using HAS3 anti-α2 antibodies showed heterodimer formation with β1 (Figure 4c).
Integrin αvβ1 heterodimers, but not α2, are mobilized to focal adhesions. (a) Double immunofluorescence with E-cadherin (ECD) or β-catenin (BCTNN) showing intercellular localization of integrin α2 (P1E6) and β1 (4B7R) subunits. Note localization of integrin αv (P2W7) subunits at cluster surfaces and cell–cell contacts. (b) After TNC and TGF-β/TNC treatments, α2 and β1 immunolocalization is dispersed into the cytoplasm of the cells after EMT change and focal adhesion staining is lacking. Scale bars: 20 μm. (c) On immunoprecipitation, an isoform of β1 with higher molecular weight was co-precipitated by α2 antibody (HAS3), without apparent change of the amount after the treatments. (d) The active β1 subunit (P4G11) is localized in small cell-substratum adhesions projected from cell clusters in the control, without αv (Q-20) colocalization (arrow heads). After TNC and TGF-β/TNC treatments, the subunits accumulated in adhesion plaques of the spreading cells on the substrata, in association with αv subunits (arrows). Scale Bar: 20 μm. (e) Immunoprecipitation of β1 showed increased heterodimers with αv subunits in both TNC- and TGF-β/TNC-treated cells, compared with the controls.
Integrin αvβ1 heterodimers are recruited to focal adhesions by TNC treatment
Integrin β1 subunits form heterodimers with αv. To detect β1 subunits in focal adhesions, we employed P4G11 antibodies that react with the Ca2+-dependent epitope and activate cell adhesion to extracellular matrix,26 suggesting recognition of an active form of β1 subunit. In the control case, immunofluorescence using P4G11 revealed small tiny adhesion plaques around cell clusters that were negative for αv (Figure 4d, arrow heads). In TNC- and TGF-β1/TNC-treated cells, P4G11 labeled some portion of αv-positive adhesion plaques (Figure 4d, arrows). On immunoprecipitation of cell lysates with P5D2 anti-β1 antibodies, almost equal amounts of α2 were co-precipitated with or without TNC treatment. In contrast, the αv contents precipitated with β1 were clearly elevated after TNC and TGF-β1/TNC treatments (Figure 4e), indicating increasing αvβ1 heterodimers and their mobilization to focal adhesions after TNC treatment.
Phosphorylation of FAK at Y925 (pY925) has been reported to be a major event in EMT of colon cancer cells.19 FAK pY925 labeling was here partially colocalized in focal adhesions with αv, β6 and β1 integrin subunits (Supplementary Figure 2), suggesting the presence of a signaling pathway for EMT mediated through these integrins.
Neutralizing antibodies inhibit TNC-induced EMT-like change
Effects of neutralizing antibodies against integrins on EMT-like change were examined in TNC-treated cells. With TNC treatment alone, addition of the neutralizing anti-αv antibody (AV1) blocked the change (Figure 5a). In the case of treatment with anti-β1 antibody P5D2, the change was also inhibited. In contrast, neutralization antibodies against αvβ6 (10D5) or α2 (P1E6) did not block EMT. More extensive EMT change was observed with the combination of TGF-β1 and TNC than with TNC alone. In TGF-β1/TNC-treated cells, AV1 treatment also inhibited the change; however, P1E6, P5D2 or 10D5 alone did not (Figure 5b). Next, we tried combined treatment of 10D5 with P1E6 or P5D2. The EMT-like change was abrogated by the combination of 10D5 with P5D2, but not with P1E6 (Figure 5c). Cellular signaling for EMT change in TGF-β1/TNC-treated cells could be independently mediated by two types of integrin heterodimers, αvβ6 and, possibly, αvβ1.
Neutralizing antibodies against αv, β1 and αvβ6 inhibit EMT. (a) Neutralizing antibodies of αv (AV1) and β1 (P5D2) integrins, but not either α2 (P1E6) or αvβ6 (10D5), inhibited EMT-like change after TNC treatment. Note that intercellular staining of β-catenin (BCTNN) is preserved in cells treated with the EMT-blocked antibodies. (b) AV1 alone also blocked EMT after the TGF-β/TNC treatment. The inset is an immunofluorescent image of β-catenin antibody labeling. (c) Combined treatment with αvβ6 (10D5) and β1 (P5D2) antibodies inhibited EMT-like change and prevented E-cadherin (ECD) delocalization after the treatment, whereas the antibodies applied singly did not. Note the stronger EMT change with TGF-β/TNC treatment than with TNC alone. Scale bars: 50 μm.
Integrin αvβ6 and αvβ1, but not α2β1, bind to TNC
We confirmed binding of αvβ6 and αvβ1 integrins to TNC immobilized on agarose beads. Several biotinylated protein bands were seen between 75 and 150 KDa in EDTA extracts (Figure 6a). By immunoblotting, integrin subunits of β1, β6 and αv were detected. Amounts of these subunits bound to TNC were increased in TGF-β/TNC-treated cells, compared with the control case (Figure 6b). Integrin α2 subunits were recognized in the lysates, but not in the EDTA extracts. As αv is the exclusive partner of β6, it could be confirmed that αvβ6 binds to TNC. To clarify a heterodimeric partner of β1, we tried addition of anti-β1 antibody in the binding assay. Addition of neutralizing antibodies P5D2 and/or 10D5 significantly decreased αv subunit bound to TNC-conjugated beads (Figure 6c), indicating binding of αvβ1 and αvβ6 heterodimers to TNC in a divalent cation-dependent manner.
Integrins αvβ1 and αvβ6, but not α2β1, bind to TNC. Biotinylated membranous proteins of MCF-7 cells were incubated with agarose beads conjugated with purified TNC. After thorough washing, TNC binding protein was extracted with 20 mM EDTA solution. Samples were analyzed by electrophoresis under non-reducing conditions, followed by staining with peroxidase conjugated streptavidin or immunoblotting. (a) Several biotinylated protein bands between 75 and 150 KDa in the EDTA extract were labeled with peroxidase-conjugated streptavidin (SA). No apparent band was visible in an eluted solution (wash) just before EDTA extraction. On immunoblot analysis of the samples, integrin subunits of αv, β6 and β1were detected. Integrin α2 subunit was recognized in the total lysates, but not in the EDTA extracts. (b) The same amounts of proteins from control and TGF-β1/TNC treated cells were applied. Integrin αv, β6 and β1 subunits bound to TNC were increased in the treated cells, compared with the control case. (c) Neutralizing antibodies P5D2 and/or 10D5 were added in the supernatants from TGF-β1/TNC treated cells. In western blotting, αv subunits bound to the beads were significantly reduced by incubation with the neutralizing antibodies, indicating that αvβ1 and αvβ6 heterodimers are TNC receptors. The band intensities were 0.70 (95% confidential intervals, 0.58–0.82) for P5D2, 0.65 (0.53–0.77) for 10D5 and 0.55 (0.24–0.86) for the combined treatment, relative to IgG controls.
Integrin subunits are expressed in human breast cancer tissues
Immunolabelling of the αv subunit was mainly found in luminal surfaces of tubular structures in large solid cancer clusters in breast cancer tissues (arrows in αv of Figure 7a), while membrane surfaces were positive in scattered cancer cells. Labeling of the β6 subunit was generally faint in the cytoplasm of clustered cells. In contrast, densely labeled cells, primarily cytoplasmic and partially membranous, were frequently observed in areas of the scattered pattern (arrows in β6 of Figure 7b). Positive β6 labeling was significantly more frequent in scattered than solid areas (P<0.0001; Table 1), and in stroma with dense TNC deposition (P<0.0001; Table 2). Immunostaining of both α2 and β1 was often evident in intercellular contacts between cancer cells in large clustered nests (arrows in α2 and β1 of Figure 7a), as well as in intraductal non-invasive components of cancers (not shown).
Immunohistochemistry of integrin subunits, αv/2 and β1/6, in solid and scatter pattern human breast cancer tissues. After immunolabelling of TNC, integrin subunits of αv/2 and β1/6 and E-cadherin, light counterstaining with hematoxylin was performed. (a) In solid pattern, large masses of breast cancer cells, TNC labeling is weak and often limited to interfaces between cancer cell clusters and the stroma. Note integrin αv subunit labeling in apical surfaces of small luminal structures (arrows) and the intercellular localization of α2 and β1 (arrows). (b) In a scattered type example, TNC is densely labeled in the stroma between/around small cancer nests and individual cells. Note the presence of integrin subunits in the cytoplasm and membrane of cancer cells to varying extents, with intense cytoplasmic and partially membranous staining of β6 (arrows). As a control, E-cadherin (ECD) is positive in both cancer types. Scale bars: 100 μm.
Discussion
The present analysis of mRNA expression of integrin subunits of αv/2/7/8/9 and β1/3/6 allowed selection of αv/2 and β1/6 as candidate subunits for TNC receptors in MCF-7 cells (Figure 1a). Expression of these subunits could also be successfully detected at the protein level by immunoblotting, whereas in the α7/8/9 and β3 cases it was very low with high Ct values. Indeed, the β3 protein was hardly detected in immunoblotting and positive adhesion plaques were essentially not observed. This is in line with a recent study showing no α9 protein on flow cytometry of MCF-7 cells, expression being closely related to the basal-like subtype of breast cancer cell.27 MCF-7 cells are luminal subtype.28 A recent study showed that α9β1 expression of colon cancer cells induces phenotypic changes consistent with EMT.24 Most breast cancer cell lines of basal-like subtype exhibit a mesenchymal phenotype,28 which may be associated with specific integrin expression including α9β1.
Integrin αvβ6 is not normally expressed in the epithelium but it is induced during wound repair,29, 30 accompanied by TNC expression.31 It is also highly upregulated in a variety of neoplastic epithelial cells,32, 33, 34 including breast cancer.35 Furthermore, expression is indicative of a poor outcome of patients with colon cancers20 and more frequent liver metastases.36 It is also associated with a poorer patient outcome in early-stage gastric cancers.37 Moreover, preferential expression is observed in invasion fronts of oral SCCs38 and in small cell clusters of colon cancers invading the stroma.20 Our β6 immunohistochemistry also showed scattered cancer cells to be more frequently positive than those forming solid masses (Figure 7 and Table 1).
Overexpression of β6 by transfection induces EMT with enhanced TNC expression in oral cancer cells.21 Another EMT model with colon cancer cells exposed to TNF-α and TGF-β1 demonstrate transcriptional upregulation of the β6 subunit and consequent induction of αvβ6 expression engaged in the phenotypic change.20 Colonic cells after TGF-β1 treatment show greater than three-fold increase in β6 expression in both the mRNA level and flow cytometry. In the present study, β6 mRNA was also dramatically upregulated by TGF-β1 (Figure 1b), but the protein amount was prominently increased only by co-stimulation with TNC (Figure 2b), being associated with EMT change. Similar results were also seen in TNC treatment of another breast cancer cells T-47D (Supplementary Figure 1). TGF-β1 alone did not increase β6 protein in immunoblotting, whereas protein expression was elevated by addition of TNC as a ligand. Regarding mRNA levels, our observations for MCF-7 cells are compatible with those for colon cancer, but inconsistency was noted with protein levels. This study showed colocalization of αv and β6 subunits in focal adhesions and dramatic increase of co-precipitated αvβ6 heterodimers after TGF-β1/TNC treatment (Figures 3b and c). Further, we could directly demonstrate αvβ6 binding to TNC (Figure 6). As MCF-7 itself does not produce TNC even after TGF-β1 stimulation,18 exogenous application of TNC may be necessary to stabilize αvβ6 proteins by binding as a ligand. TGF-β is generally an inducer of TNC synthesis in both epithelial and mesenchymal cells. Expression of TNC in colon cancer cells after TGF-β1 addition therefore should be examined.
Immunohistochemistry of oral SCC demonstrated β6 labeling at the epithelial–connective tissue interfaces, associated with TNC staining.39 In late stages of oral mucosal healing, β6 expression in the basal cells temporarily and spatially coincides with TNC expression in epithelial cells and granulation tissue beneath the repairing epithelium.31 In bullous keratopathy corneas, β6 expression specifically correlates with the deposition of subepithelial TNC, more than with fibronectin or vitronectin, other αvβ6 ligands.40 We also demonstrated β6-positive cancer cells to be more frequent in TNC-positive areas in breast cancer tissues (Table 2). Our observation of stabilization of β6 protein by TNC could account for the close association between TNC and αvβ6 integrin. In addition, as αvβ6 integrin can activate latent TGF-β,41, 42 release of TGF-β from the latency-associated protein may stimulate expression of both TNC and β6 subunits by epithelial (cancer) cells and of TNC by adjacent stromal fibroblasts, forming a loop of amplified TGF-β signals.
Neutralizing antibodies 10D5 against αvβ6 and P5D2 for β1 cooperated in inhibiting EMT under TGF-β1/TNC treatment (Figure 5c). The experiments clarified that integrins actually contribute to EMT signaling after TGF-β1/TNC treatment. Integrin αvβ6 binds to TNC and mediates the signaling, but another integrin containing β1 independently acts in the EMT induction. We speculated that αvβ1 integrin as well as αvβ6 is conducive for TNC-induced EMT. Integrin αvβ1 is an RGD-dependent receptor for fibronectin, vitronectin43 and osteopontin.44 It has been suggested that TNC is also a ligand, albeit with limited direct evidence. Migration of oligodendrocyte precursors on astroglial matrices rich in TNC is inhibited by anti-αvβ1 antibodies.45 Adhesion of glioma U251.3 cells to TNC was found to be completely blocked by combined treatment with αv and β1 antibodies.46 Epithelial cells also express αvβ1 heterodimers.47 In this study, αv subunits co-precipitated by β1 antibodies were increased in TNC- and TGF-β1/TNC-treated cells, colocalizing in focal adhesions (Figures 4d and e). EMT induced by TNC alone was inhibited by P5D2 or AV1 alone (Figure 5a). Furthermore, addition of the neutralizing antibody reduced αv subunits in the binding assay to TNC, supporting heterodimer formation of the subunits binding to TNC (Figure 6c). These findings provide the first direct evidence that αvβ1 heterodimers act as a receptor for TNC, constituting a signaling pathway to evoke EMT.
Integrin α2β1 induces collagen-I mediated EMT-like change of pancreatic cancer cells through FAK phosphorylation, cooperating with DDR1.25 Integrin α2β1 also binds to TNC, and contributes to attachment and spreading on endothelial cells.48 This integrin is also responsible for cell adhesion and migration of cancer cells on TNC.49 In both these earlier experiments, the neutralizing anti-α2 antibody P1E6 inhibited TNC functions. Here, P1E6 did not block EMT change (Figure 6) and affinity binding of α2 for TNC could not be observed (Figure 5), indicating no involvement of α2β1, at least, in induction of EMT in MCF-7 cells.
We have shown clearly that αvβ1 and αvβ6 integrins can independently participate in TNC-induced EMT-like change. Additional exposure to αvβ6 with αvβ1 may be responsible for the stronger phenotypic change of TGF-β1/TNC-treated cells than with TNC alone. We can conclude that these integrins transduce EMT signals from the TNC-rich environment of cancer tissues. Integrin αvβ6 may promote migration, generate signals for proliferation and protection from anoikis, activate latent TGF-βs and upregulate matrix metalloproteinase expression.32, 33, 34 The interaction of αvβ6 with TNC may yield signals for more malignant behavior of cancer cells including EMT. Further investigations are warranted to clarify their interrelationships in a variety of pathological states. In addition, functions of the αvβ1 integrin in cancer progression, much less explored than αvβ6, are clearly of interest.
Materials and methods
Antibodies
Antibodies used in this study are summarized in Table 3.
Cell culture, EMT induction and blocking by antibodies
MCF-7 cells and T-47D were cultured as previously described.18 After seeding at 5 × 104 cells per well in 12-well plates (BD Falcon, Franklin Lakes, NJ, USA), 10 μg/ml of TNC purified from conditioned medium of U-251MG8 was added to the medium. After overnight incubation, 5 ng/ml of TGF-β1 (Roche Diagnostics, Mannheim, Germany) was added and the cells were cultured for a further 48 h. Induction of EMT-like change of T-47D was assessed as previously described.18
Neutralizing antibodies (5 μg/ml) for αvβ6, α2 and β1 were added to the medium when the cells were plated. The anti-αv antibody was added at 1:40 after overnight incubation. As a negative control, protein A-purified monoclonal antibody against a paramyxovirus protein (IgG1κ) was used.
Quantitative reverse-transcription PCR
Expression of mRNAs for integrin subunits αv/2/7/8/9 and β1/β3/6 was quantified by qPCR. Total RNA was extracted using the ISOGEN reagent (Nippon Gene, Tokyo, Japan). Total RNA (1 μg) was reverse-transcribed using a cDNA synthesis kit (Roche Diagnostics) with anchored-oligo (dT)18 primers. Quantitative PCR was performed using a LightCycler real-time PCR instrument and an SYBR Green I Master kit (Roche Diagnostics). QuantiTect primers for the subunits and GAPDH were purchased from Qiagen (Valencia, CA, USA). The reactions were performed at 95 °C for 10 min to activate the polymerase, followed by 40 cycles at 95 °C/5 s, 55 °C/10 s and 72 °C/20 s. The specificity of the PCR reactions was confirmed with a heat dissociation protocol (from 60–95 °C). The PCR cycle threshold (Ct) values were determined with the second derivative maximum method using LightCycler Software. Quantitative analyses of expression changes of integrin mRNAs after the treatments were performed at least in triplicate.
Immunoblotting
Cells were lysed using a lysis buffer (1% SDS, 50 mM Tris–HCl buffer, pH 6.8). The amount of protein in each extract was evaluated by bicinchoninic acid assay (BCA, Pierce, Rockford, IL, USA). Equal amounts of total protein were mixed with Laemmli’s sample buffer without 2-mercaptoethanol and electrophoresed on 5–20% polyacrylamide gradient gels. The proteins were electrically transferred to Immobilon membranes (Millipore, Billerica, MA, USA), and labeled with primary antibodies against integrin αv/2, β1/3/5/6 and α-tubulin as an intrinsic control, followed by exposure to peroxidase-labeled anti-mouse IgG (3000–5000-fold diluted; Bio-Rad, Hercules, CA, USA) or anti-rabbit IgG (2000-fold diluted; Sigma-Aldrich, St Louis, MO, USA) and visualization with ECL Plus (GE Healthcare, Waukesha, WI, USA). First antibodies were used at 1000–3000-fold dilutions. Band intensities were quantified using Image J and the values were normalized to intensities of α-tubulin bands. All quantitative analyses were performed at least in triplicate.
Immunofluorescence
MCF-7 cells were grown on cover glasses and fixed in 2% paraformaldehyde/PBS with 0.2% Triton X-100 for 10 min. After treatment with 10% normal goat serum, they were exposed to primary antibodies against E-cadherin, β-catenin, αv/2 and β1/3/5/6. Subsequently, the cells were treated with appropriate secondary antibodies, fluorescein-labeled goat anti-mouse IgG or anti-rabbit IgG (200-fold diluted; MBL, Nagoya, Japan). For double immunofluorescence, rhodamine-labeled goat anti-mouse IgG (Tago, Burlingame, CA, USA) was used. For P4G11, 1 mM CaCl2 was included in all solutions.26 Observation was performed under an epifluorescence microscope with appropriate filter sets and a 40 × or 60 × objective lens (Olympus, Tokyo, Japan), and photographs were taken using a cooled CCD camera.
Immunoprecipitation
Cells were lysed in ice-cold lysis buffer, 1% Nonidet P-40, 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM CaCl2 and 1 mM MgCl2 with protease inhibitors (complete mini EDTA-free, Roche Diagnostics). The lysates were centrifuged at 15 000 × g for 20 min and supernatants were pre-cleared with protein G beads (Sigma-Aldrich) for 30 min, then incubated with antibodies against αv, α2 and β1, or mouse IgG as a negative control, at 4 °C for 1 h. Complexes were precipitated with protein G beads for 2 h. The beads were then washed three times with the lysis buffer and boiled with sample buffer. The samples were subjected to immunoblotting, employing primary antibodies of rabbit origin. Representative data are shown from at least three independent experiments that yielded similar results.
Binding of integrins to TNC
Cell surface proteins were biotinylated by incubating the cells with 0.5 mg/0.5 ml of sulfo-NHS-biotin (Pierce) in PBS at 4 °C for 1 h. After several washes, biotinylated cells were lysed with 0.5 ml of extraction buffer (50 mM Tris-HCl, pH 7.6, 50 mM NaCl, 100 mM n-octyl-β -D-glucoside (Dotite, Kumamoto, Japan), 1 mM MgCl2, 2 mM MnCl2, plus protease inhibitor cocktail) for 20 min at 4 °C. Cell debris was removed by centrifugation, and the supernatants were pre-cleared by incubation for 1 h with agarose beads. Purified TNC (0.5 mg) was coupled to 1 ml of NHS-activated agarose (Pierce). TNC-conjugated beads (100 μl) were added into the pre-cleared supernatants, and the slurry was incubated with vigorous agitation at 4 °C overnight. The beads were subsequently extensively washed with extraction buffer, and sequentially with the buffer containing 0.2 M NaCl twice. The binding proteins were eluted by 100 μl of the extraction buffer without divalent cations supplemented with 20 mM EDTA. Samples were analyzed by staining with peroxidase-conjugated streptavidin (2000-fold diluted, GE Healthcare) or immunoblotting. Neutralizing antibody P5D2 or 10D5 (1.5 μg) was added into the pre-cleared supernatants, followed by 1-h incubation and then addition of TNC beads.
Immunohistochemistry
Immunohistochemical analysis was performed on 35 cases of fully invasive ductal carcinoma of the breast using archival samples for pathological diagnoses that had been fixed in formalin and routinely processed for embedding in paraffin. Use of the samples was approved by written informed consent from the patients under a protocol authorized by the ethical committee of our university. The same samples and immunohistochemical techniques had been used in our previous paper.18 Sections were treated with Super Block solution (Scytek Laboratories, Logan, UT, USA) before incubation with primary antibodies against TNC, αv/2, β1/6 and E-cadherin overnight. All primary antibodies were used at a concentration of 0.5 μg/ml or a 1000-fold dilution. After being washed, sections were treated with a commercially available polyvalent LSAB kit (Scytek), followed by color development with 3,3′-diaminobenzidine/H2O2 solution. The invasive patterns of ductal carcinomas at the peripheral margins of the tumours were classified into two groups: solid and scattered. A maximum of three representative areas of each type were selected in each breast cancer specimen, and immunoreactivity was assessed in 63 solid- and 84 scattered-type areas. Immunolabelling of β6 subunits in cancer cells stronger than that in normal epithelium was defined as positive. The relation between TNC and β6 positivity was examined in the same fields of adjacent serial sections.
Statistical analyses
Results are presented as means±s.d’s. Statistical analysis was performed by one-way analysis of variance with the Bonferroni means comparison test. Differences in TNC and β6 staining with reference to histological patterns and between TNC and β6 labeling were assessed by Fisher’s exact test. P<0.05 was considered statistically significant.
Abbreviations
- EMT:
-
epithelial–mesenchymal transition
- FAK:
-
focal adhesion kinase
- LSAB:
-
labeled streptavidin biotin
- NHS:
-
N-hydroxysuccinimide
- qPCR:
-
quantitative PCR
- SCC:
-
squamous cell carcinoma
- TGF:
-
transforming growth factor
- TNC:
-
tenascin-C
- TNF:
-
tumor necrosis factor
References
Orend G, Chiquet-Ehrismann R . Tenascin-C induced signaling in cancer. Cancer Lett 2006; 244: 143–163.
Midwood KS, Orend G . The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun Signal 2009; 3: 287–310.
Midwood KS, Hussenet T, Langlois B, Orend G . Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68: 3175–3199.
Yoshida T, Matsumoto E, Hanamura N, Kalembeyi I, Katsuta K, Ishihara A et al. Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 1997; 182: 421–428.
Ishihara A, Yoshida T, Tamaki H, Sakakura T . Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res 1995; 1: 1035–1041.
Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008; 451: 147–152.
Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011; 17: 867–874.
Yoshida T, Yoshimura E, Numata H, Sakakura Y, Sakakura T . Involvement of tenascin-C in proliferation and migration of laryngeal carcinoma cells. Virchows Arch 1999; 435: 496–500.
Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R et al. Involvement of large tenascin-C splice variants in breast cancer progression. Am J Pathol 2003; 162: 1857–1867.
Calvo A, Catena R, Noble MS, Carbott D, Gil-Bazo I, Gonzalez-Moreno O et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene 2008; 27: 5373–5384.
Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS et al. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res 2009; 11: R24.
Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T . Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor beta1. Int J Cancer 2003; 105: 53–60.
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA . Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119: 1438–1449.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Jahkola T, Toivonen T, von Smitten K, Blomqvist C, Virtanen I . Expression of tenascin in invasion border of early breast cancer correlates with higher risk of distant metastasis. Int J Cancer 1996; 69: 445–447.
Jahkola T, Toivonen T, Virtanen I, Von Smitten K, Nordling S, von Boguslawski K et al. Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 1998; 78: 1507–1513.
Jahkola T, Toivonen T, Nordling S, von Smitten K, Virtanen I . Expression of tenascin-C in intraductal carcinoma of human breast: relationship to invasion. Eur J Cancer 1998; 34: 1687–1692.
Nagaharu K, Zhang X, Yoshida T, Katoh D, Hanamura N, Kozuka Y et al. Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am J Pathol 2011; 178: 754–763.
Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat Cell Biol 2002; 4: 632–638.
Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 2005; 115: 339–347.
Ramos DM, Dang D, Sadler S . The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res 2009; 29: 125–130.
Richter P, Umbreit C, Franz M, Berndt A, Grimm S, Uecker A et al. EGF/TGFβ1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J Oral Pathol Med 2011; 40: 46–54.
Galliher AJ, Schiemann WP . Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 2006; 8: R42.
Gupta SK, Oommen S, Aubry MC, Williams BP, Vlahakis NE . Integrin α9β1 promotes malignant tumor growth and metastasis by potentiating epithelial-mesenchymal transition. Oncogene 2013; 32: 141–150.
Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR . Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol 2008; 180: 1277–1289.
Wayner EA, Gil SG, Murphy GF, Wilke MS, Carter WG . Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J. Cell Biol 1993; 121: 1141–1152.
Allen MD, Vaziri R, Green M, Chelala C, Brentnall AR, Dreger S et al. Clinical and functional significance of α9β1 integrin expression in breast cancer: a novel cell-surface marker of the basal phenotype that promotes tumour cell invasion. J Pathol 2011; 223: 646–658.
Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis 2008; 25: 629–642.
Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF et al. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995; 108: 2241–2251.
Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T et al. Keratinocytes in human wounds express αvβ6 integrin. J Invest Dermatol 1996; 106: 42–48.
Häkkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H . Immunolocalization of tenascin-C, alpha9 integrin subunit, and alphavbeta6 integrin during wound healing in human oral mucosa. J Histochem Cytochem 2000; 48: 985–998.
Bandyopadhyay A, Raghavan S . Defining the role of integrin αvβ6 in cancer. Curr Drug Targets 2009; 10: 645–652.
Thomas GJ, Nystrom ML, Marshall JF . Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med 2006; 35: 1–10.
Eberlein C, Kendrew J, McDaid K, Alfred A, Kang JS, Jacobs VN et al. A human monoclonal antibody 264RAD targeting αvβ6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene (e-pub ahead of print 29 October 2012; doi:10.1038/onc.2012.460.
Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y . Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer (Tokyo, Japan) 2000; 7: 19–26.
Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS et al. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci 2008; 99: 879–887.
Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W, Wang JY et al. Integrin alphavbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol 2008; 20: 61–66.
Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H et al. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol 2004; 202: 14–22.
Regezi JA, Ramos DM, Pytela R, Dekker NP, Jordan RC . Tenascin and beta 6 integrin are overexpressed in floor of mouth in situ carcinomas and invasive squamous cell carcinomas. Oral Oncol 2002; 38: 332–336.
Ljubimov AV, Saghizadeh M, Pytela R, Sheppard D, Kenney MC . Increased expression of tenascin-C-binding epithelial integrins in human bullous keratopathy corneas. J Histochem Cytochem 2001; 49: 1341–1350.
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96: 319–328.
Wipff PJ, Hinz B . Integrins and the activation of latent transforming growth factor beta1-An intimate relationship. Eur J Cell Biol 2008; 87: 601–615.
Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E . A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem 1990; 265: 5934–5937.
Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW . A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem 1995; 270: 26232–26238.
Milner R, Edwards G, Streuli C, Ffrench-Constant C . A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci 1996; 16: 7240–7252.
Deryugina EI, Bourdon MA . Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci 1996; 109: 643–652.
Koivisto L, Grenman R, Heino J, Larjava H . Integrins alpha5beta1, alphavbeta1, and alphavbeta6 collaborate in squamous carcinoma cell spreading and migration on fibronectin. Exp Cell Res 2000; 255: 10–17.
Sriramarao P, Mendler M, Bourdon MA . Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci 1993; 105: 1001–1012.
Ramos DM, Chen BL, Boylen K, Stern M, Kramer RH, Sheppard D et al. Stromal fibroblasts influence oral squamous cell carcinoma cell interactions with tenascin-C. Int J Cancer 1997; 72: 369–376.
Acknowledgements
We thank Drs T. Ogawa and T. Shiraishi for collection of breast cancer samples and helpful discussions. DK and KN were supported by the program for undergraduate research training of Mie University School of Medicine. This study was also supported by grants (#21590368 and #24390087) to TY from Ministry of Education, Culture, Sports, Science and Technology-Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TY receives royalties on TNC antibodies from Immuno-Biological Lab, Japan. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogenesis website .
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Katoh, D., Nagaharu, K., Shimojo, N. et al. Binding of αvβ1 and αvβ6 integrins to tenascin-C induces epithelial–mesenchymal transition-like change of breast cancer cells. Oncogenesis 2, e65 (2013). https://doi.org/10.1038/oncsis.2013.27
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2013.27