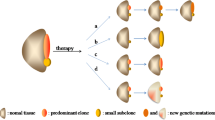
Key Points
-
Steady progress in the development of protocol-based therapy has led to a cure rate of more than 80% in children and up to 40% in adults with acute lymphoblastic leukaemia (ALL). However, innovative approaches to therapy are needed to further improve the cure rates and to reduce the toxic side effects of current intensive regimens.
-
Most of the conventional drugs used to treat leukaemia are nonspecific and work either by targeting DNA directly, inhibiting nucleic acid synthesis, blocking protein synthesis or interfering with the mitotic spindle apparatus. These, therefore, have a narrow therapeutic index.
-
Strategies to improve delivery and therapeutic index of conventional drugs include the encapsulation of drugs into liposomes or binding drugs covalently to monomethoxy-polyethylene glycol.
-
Newer antifolates have been designed to improve membrane support, and/or circumvent resistance due to impaired polyglutamation or inability to deplete reduced folate pools.
-
Newer nucleoside analogues have been developed to improve bioavailability and to decrease neurotoxicity.
-
Monoclonal antibodies, targeted towards leukaemia-associated antigens, have been administered in the unconjugated forms or conjugated to antileukaemic drugs, immunotoxins or radioactive molecules.
-
Molecular therapeutic agents have been developed to inhibit certain key enzymes, such as tyrosine kinases, DNA methyltransferases, histone deacetylases, γ-secretase, serine and threonine kinase and proteosomes.
-
As strategies to analyse the molecular genetic and epigenetic aberrations in cancer cells yield ever greater insights into ALL pathogenesis, one can expect an expanding repertoire of targeted therapies. This might result in an era of individualized molecular medicine and lead to more effective and less toxic regimens.
Abstract
Although contemporary treatments cure more than 80% of children with acute lymphoblastic leukaemia (ALL), some patients require intensive treatment and many patients still develop serious acute and late complications owing to the side effects of the treatments. Furthermore, the survival rate for adults with ALL remains below 40%. Therefore, new treatment strategies are needed to improve not only the cure rate but also the quality of life of these patients. Here, we discuss emerging new treatments that might improve the clinical outcome of patients with ALL. These include new formulations of existing chemotherapeutic agents, new antimetabolites and nucleoside analogues, monoclonal antibodies against leukaemia-associated antigens, and molecular therapies that target genetic abnormalities of the leukaemic cells and their affected signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pui, C. H. & Evans, W. E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354, 166–178 (2006). A comprehensive review of the central role leukaemia biology and host factors have in the design of effective therapies for ALL.
Oeffinger, K. C. & Hudson, M. M. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J. Clin. 54, 208–236 (2004).
Pui, C. H. et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N. Engl. J. Med. 349, 640–649 (2003).
Pui, C. H., Relling, M. V. & Downing, J. R. Acute lymphoblastic leukemia. N. Eng. J. Med. 350, 1535–1548 (2004). A review of the pathobiology of ALL, emphasizing results that are likely to have the greatest impact on clinical management in the coming decade.
Armstrong, S. A. & Look, A. T. Molecular genetics of acute lymphoblastic leukemia. J. Clin. Oncol. 23, 6306–6315 (2005). Informative review of the signal-transduction pathways in ALL, and the role gene expression has in the classification of ALL and the design of molecularly targeted therapy.
LeBien, T. W. Fates of human B-cell precursors. Blood 96, 9–23 (2000).
Gill, J. et al. Thymic generation and regeneration. Immunol. Rev. 195, 28–50 (2003).
Pui, C. H. & Evans, W. E. Acute lymphoblastic leukemia. N. Engl. J. Med. 339, 605–615 (1998).
Pui, C. H., Campana, D. & Evans, W. E. Childhood acute lymphoblastic leukaemia — current status and future perspectives. Lancet Oncol. 2, 597–607 (2001).
Ferrando, A. A. et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 1, 75–87 (2002).
Yeoh, E. J. et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 1, 133–143 (2002).
Armstrong, S. A. et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nature Genet. 30, 41–47 (2002).
Haferlach, T. et al. Global approach to the diagnosis of leukemia using gene expression profiling. Blood 106, 1189–1198 (2005).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005). This report describes a new, bead-based flow cytometric microRNA-expression-profiling method, and highlights the potential of microRNA in cancer diagnosis.
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004). A report on the presence of activating mutations in more than 50% of human T-cell ALL, providing a strong rationale for targeted therapies that interfere with NOTCH signalling.
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell. 2, 103–112 (2002).
Krug, U., Ganser, A. & Koeffler, H. P. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene 21, 3475–3495 (2002).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nature Rev. Cancer 2, 594–604 (2002).
Avramis, V. I. et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood 99, 1986–1994 (2002).
Abshire, T. C., Pollock, B. H., Billett, A. L., Bradley, P. & Buchanan, G. R. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood 96, 1709–1715 (2000).
Hak, L. J. et al. Asparaginase pharmacodynamics differ by formulation among children with newly diagnosed acute lymphoblastic leukemia. Leukemia 18, 1072–1077 (2004).
Moghrabi, A. et al. Results of the Dana–Farber Cancer Institute ALL Consortium protocol 95-01 for children with acute lymphoblastic leukemia. Blood 26 Sep 2006 (doi:10.1182/blood-2006-06-027714 ).
Bomgaars, L. et al. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J. Clin. Oncol. 22, 3916–3921 (2004).
Glantz, M. J. et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J. Clin. Oncol. 17, 3110–3116 (1999).
Jabbour, E. et al. Neurological complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood 5 Jan 2007 (doi:10.1182/blood-2006-08-043646).
Offidani, M. et al. Comparison of two regimens for the treatment of elderly patients with acute lymphoblastic leukemia (ALL). Leuk. Lymphoma 46, 233–238 (2005).
Consoli, U. et al. The novel anthracycline annamycin is not affected by P-glycoprotein-related multidrug resistance: comparison with idarubicin and doxorubicin in HL-60 leukemia cell lines. Blood 88, 633–644 (1996).
Zou, Y., Priebe, W., Stephens, L. C. & Perez-Soler, R. Preclinical toxicity of liposome-incorporated annamycin: selective bone marrow toxicity with lack of cardiotoxicity. Clin. Cancer Res. 1, 1369–1374 (1995).
Boman, N. L., Bally, M. B., Cullis, P. R., Mayer, L. D. & Webb, M. S. Encapsulation of vincristine in liposomes reduces its toxicity and improves its antitumor efficacy. J. Liposome Res. 5, 523–541 (1995).
Gelmon, K. A. et al. Phase I study of liposomal vincristine. J. Clin. Oncol. 17, 697–705 (1999).
Thomas, D. A. et al. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer 106, 120–127 (2006).
Walling, J. From methotrexate to premetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Invest. New Drugs 24, 37–77 (2006).
Sarris, A. H. et al. Trimetrexate in relapsed T-cell lymphoma with skin involvement. J. Clin. Oncol. 20, 2876–2880 (2002).
O'Connor, O. et al. Pralatrexate (10-propargyl-10-deazaaminopterin (PDX), a novel antifolate, effects durable complete remissions (CR) in patients with a diversity of drug resistant T-cell lymphomas with minimal toxicity. Blood 106, 752a (2005).
Wang, E. S., Connor, O., She, Y., Zelenetz, A. D., Sirotnak, F. M. & Moore, M. A. Activity of a novel anti-folate (PDX, 10-propargyl-10-deazaaminoptin) against human lymphoma is superior to methotrexate and correlates with tumor RFC-1 gene expression. Leuk. Lymphoma 44, 1027–1035 (2003).
Toner, L. E. et al. The schedule-dependent effects of the novel antifolate pralatrexate and gemcitabine are superior to methotrexate and cytarabine in models of human non-Hodgkin's lymphoma. Clin. Cancer Res. 12, 924–932 (2006).
Villela, L. R., Stanford, B. L. & Shah, S. R. Pemetrexed, a novel antifolate therapeutic alternative for cancer chemotherapy. Pharmacotherapy 26, 641–654 (2006).
Parker, W. B. et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerase by its 5′-triphosphate. Cancer Res. 51, 2386–2394 (1991).
Santana, V. M. et al. A phase I clinical trial of 2-chlorodeoxyadenosine in pediatric patients with acute leukemia. J. Clin. Oncol. 9, 416–422 (1991).
Kornblau, S. M. et al. Clinical and laboratory studies of 2-chlorodeoxyadenosine +/− cytosine arabinoside for relapsed or refractory acute myelogenous leukemia in adults. Leukemia 10, 1563–1569 (1996).
Van Den Neste, E. et al. 2-Chlorodeoxyadenosine with or without daunorubicin in relapsed or refractory acute myeloid leukemia. Ann. Hematol. 76, 19–23 (1998).
Holowiecki, J. et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 18, 989–997 (2004).
Vahdat, L. et al. Therapeutic and neurotoxic effects of 2-chlorodeoxyadenosine in adults with acute myeloid leukemia. Blood 84, 3429–3434 (1994).
Warrell, R. P. Jr & Berman, E. Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. J. Clin. Oncol. 4, 74–79 (1986).
Montgomery, J. A., Shortnacy-Fowler, A. T., Clayton, S. D., Riordan, J. M. & Secrist, J. A. 3rd. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-β-D-arabinofuranosyladenine. J. Med. Chem. 35, 397–401 (1992).
Avramis, V. I. & Plunkett, W. 2-fluoro-ATP: a toxic metabolite of 9-β-D-arabinosyl-2-fluoroadenine. Biochem. Biophys. Res. Comm. 113, 35–43 (1983).
Kantarjian, H. M. et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J. Clin. Oncol. 21, 1167–1173 (2003).
Kantarjian, H. et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood 102, 2379–2386 (2003).
Jeha, S. et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood 103, 784–789 (2004).
Jeha, S. et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J. Clin. Oncol. 24, 1917–1923 (2006). References 49 and 50 report the paediatric trials that led to the accelerated clofarabine approval to treat children with ALL whose disease had not responded to or had relapsed following at least two prior regimens.
Giblett, E. R., Ammann, A. J., Wara, D. W., Sandman, R. & Diamond, L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet 1, 1010–1013 (1975).
Cohen, A., Lee, J. W. & Gelfand, E. W. Selective toxicity of deoxyguanosine and arabinosylguanine for T-leukemic cells. Blood 61, 660–666 (1983).
Reist, E. J. & Goodman, L. Synthesis of 9-β-D-arabinofuranosylguanine. Biochemistry 3, 15–18 (1964).
Kurtzberg, J. et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J. Clin. Oncol. 23, 3396–3403 (2005).
Berg, S. L. et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J. Clin. Oncol. 23, 3376–3382 (2005). References 54 and 55 report the trials that led to the accelerated nelarabine approval to treat adults and children with T-cell ALL and T-cell lymphoblastic lymphoma whose disease had not responded to or had relapsed following at least two prior regimens.
Miles, R. W., Tyler, P. C., Furneaux, R. H., Bagdassarian, C. K. & Schramm, V. L. One-third-the-sites transition-state inhibitors for purine nucleoside phosphorylase. Biochemistry 37, 8615–8641 (1998).
Bantia, S. et al. Purine nucleoside phosphorylase inhibitor BCX-1777 (Immucillin-H) — a novel potent and orally active immunosuppressive agent. Int. Immunopharm. 1, 1199–1210 (2001).
Gandhi, V. et al. A proof-of-principle pharmacokinetic, pharmacodynamic, and clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777, forodesine). Blood 106, 4253–4260 (2005).
Schulz, H. et al. Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematol. 89, 753–754 (2004).
Stasi, R., Pagano, A., Stipa, E. & Amadori, S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood 98, 952–957 (2001).
Leandro, M. J., Edwards, J. C., Cambridge, G., Ehrenstein, M. R. & Isenberg, D. A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 46, 2673–2677 (2002).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Thomas, D. A. et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 106, 1569–1580 (2006).
Thomas, D. A. et al. Update of the modified hyper-CVAD regimen in newly diagnosed adult acute lymphocytic leukemia (ALL). Blood 102, 880a (2003).
Jeha, S. et al. Prognostic significance of CD20 expression in childhood B-cell precursor acute lymphoblastic leukemia. Blood 108, 3302–3304 (2006).
Dillman, R. O. et al. Radioimmunotherapy of B-cell lymphoma with radiolabelled anti-CD20 monoclonal antibodies. Clin. Exp. Med. 6, 1–12 (2006).
Leonard, J. P. et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin. Cancer Res. 10, 5327–5334 (2004).
Stein, R. et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma. Clin. Cancer Res. 10, 2868–2878 (2004).
Leonard, J. P. et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J. Clin. Oncol. 23, 5044–5051 (2005).
Linden, O. et al. Dose-fractionated radioimmunotherapy in non-Hodgkin's lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab. Clin. Cancer Res. 11, 5215–5222 (2005).
Uckun, F. M. et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood 71, 13–29 (1988).
Goulet, A. C. et al. Conjugation of blocked ricin to an anti-CD19 monoclonal antibody increases antibody-induced cell calcium mobilization and CD19 internalization. Blood 90, 2364–2375 (1997).
Stone, M. J. et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood 88, 1188–1197 (1996).
Bae, J., Martinson, J. A. & Klingemann, H. G. Identification of CD19 and CD20 peptides for induction of antigen-specific CTLs against B-cell malignancies. Clin. Cancer Res. 11, 1629–1638 (2005).
Vallera, D. A. et al. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin. Cancer Res. 11, 3879–3888 (2005).
Gilleece, M. H. & Dexter, T. M. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood 82, 807–812 (1993).
Piccaluga, P. P. et al. Anti-leukemic and anti-GVHD effects of Campath-1H in acute lymphoblatic leukemia relapsed after stem-cell transplantation. Leuk. Lymphoma 45, 731–733 (2004).
Laporte, J. P. et al. Remission of adult acute lymphocytic leukaemia with alemtuzumab. Leukemia 18, 1557–1558 (2004).
Tibes, R. et al. Activity of alemtuzumab in patients with CD52-positive acute leukemia. Cancer 106, 2645–2651 (2006).
Stock, W. et al. Incorporation of alemtuzumab into front-line therapy of adult acute lymphoblastic leukemia (ALL) is feasible: a phase I/II study from the Cancer and Leukemia Group B (CALGB 10102). Blood 106, 46a (2005).
Sievers, E. L. et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 19, 3244–3254 (2001).
Arceci, R. J. et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 106, 1183–1188 (2005).
Lo-Coco, F. et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood 104, 1995–1999 (2004).
Golay, J. et al. Gemtuzumab ozogamicin (Mylotarg) has therapeutic activity against CD33 acute lymphoblastic leukaemias in vitro and in vivo. Br. J. Haematol. 128, 310–317 (2005).
Zwaan, C. M. et al. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: first clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia 17, 468–470 (2003). References 84 and 85 describe in vitro, in vivo and clinical activity of gemtuzumab ozogamycin against CD33+ ALL.
Cotter, M., Rooney, S., O'Marcaigh, A. & Smith, O. P. Successful use of gemtuzumab ozogamicin in a child with relapsed CD33-positive acute lymphoblastic leukaemia. Br. J. Haematol. 122, 687–688 (2003).
Balduzzi, A. et al. Molecular remission induced by gemtuzumab ozogamicin associated with donor lymphocyte infusions in t(4;11) acute lymphoblastic leukemia relapsed after transplantation. Leukemia 17, 2247–2248 (2003).
Jedama, I. et al. Internalization and cell cycle-dependent killing of leukemia cells by gemtuzumab ozogamicin: rational for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia 18, 316–325 (2004).
Graux, C. et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nature Genet. 36, 1084–1089 (2004).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR–ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Champagne, M. A. et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children's Oncology Group phase 1 study. Blood 104, 2655–2660 (2004).
Thomas, D. A. et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 103, 4396–4407 (2004).
Towatari, M. et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR–ABL-positive acute lymphoblastic leukemia. Blood 104, 3507–3512 (2004). References 90 and 91 report the activity of imatinib when used as a single agent in patients with relapsed Ph+ ALL. References 92 and 93 report on the safety and efficacy of administering imatinib in combination with intensive chemotherapy regimens in patients newly diagnosed with Ph+ ALL.
Rea, D. et al. High-dose imatinib mesylate combined with vincristine and dexamethasone (DIV regimen) as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia 20, 400–403 (2006).
Lee, S. et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 105, 3449–3457 (2005).
Wassmann, B. et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 106, 458–463 (2005).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science 293, 876–880 (2001).
Roche-Lestienne, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood 100, 1014–1018 (2002).
O'Hare, T. et al. In vitro activity of Bcr–Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 65, 4500–4505 (2005).
Kantarjian, H. et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 354, 2542–2551 (2006).
Talpaz, M. et al. Dasatinib in imatinib-resistant Philadelphia chromosome positive leukemias. N. Engl. J. Med. 354, 2531–2541 (2006).
Quintas-Cardama, A. et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogeneous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood 21 Sep 2006 (doi:10.1182/blood-2006-07-035493).
Giles, F. J. et al. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR–ABL mutation. Blood 21 Sep 2006 (doi:10.1182/blood-2006-05-025049).
Lee, B. H. et al. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene 24, 7882–7892 (2005).
Smith, B. D. et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 103, 3669–3676 (2004).
Fiedler, W. et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105, 986–993 (2005).
Armstrong, S. A. et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell 3, 173–183 (2003).
Torelli, G. F. et al. FLT3 inhibition in t(4;11)+ adult acute lymphoid leukaemia. Br. J. Haematol. 130, 43–50 (2005).
Brown, P. et al. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood 105, 812–820 (2005).
Levis, M., Pham, R., Smith, B. D. & Small, D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 104, 1145–1150 (2004).
Piloto, O. et al. IMC-EB10, an anti-FLT3 monoclonal antibody, prolongs survival and reduces nonobese diabetic/severe combined immunodeficient engraftment of some acute lymphoblastic leukemia cell lines and primary leukemic samples. Cancer Res. 66, 4843–4851 (2006).
Hoover, R. R., Mahon, F. X., Melo, J. V. & Daley, G. Q. Overcoming STI571 resistance with the farnesyl transferase inhibitor SCH66336. Blood 100, 1068–1071 (2002).
Kurzrock, R. et al. Phase II study of R115777, a farnesyl transferase inhibitor, in myelodysplastic syndrome. J. Clin. Oncol. 22, 1287–1292 (2004).
Borthakur, G. et al. Pilot study of lonafarnib, a farnesyl transferase inhibitor, in patients with chronic myeloid leukemia in the chronic or accelerated phase that is resistant or refractory to imatinib therapy. Cancer 106, 346–352 (2006).
Bueso-Ramos, C. et al. Protein expression of a triad of frequently methylated genes, p73, p57Kip2, and p15, has prognostic value in adult acute lymphoblastic leukemia independently of its methylated status. J. Clin. Oncol. 23, 3932–3939 (2005).
Zheng, S. et al. Hypermethylation of the 5′ CpG island of the FHIT gene is associated with hyperdiploid and translocation-negative subtypes of pediatric leukemia. Cancer Res. 64, 2000–2006 (2004).
Stam, R. W. et al. Silencing of the tumor suppressor gene FHIT is highly characteristic for MLL gene rearranged infant acute lymphoblastic leukemia. Leukemia 20, 264–271 (2006).
Christman, J. K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495 (2002).
Wijermans, P. et al. Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J. Clin. Oncol. 18, 956–962 (2000).
Klisovic, M. I. et al. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia 17, 350–358 (2003).
Messinger, Y, Reaman, G. H., EK, O. & Uckun, F. M. Evaluation of temozolomide in a SCID mouse model of human B-cell precursor leukemia. Leuk. Lymphoma 33, 289–293 (1999).
Seiter, K. et al. Phase I study of temozolomide in relapsed/refractory acute leukemia. J. Clin. Oncol. 20, 3249–3253 (2002).
Khan, R. B., Raizer, J. J., Malkin, M. G., Bazylewicz, K. A. & Abrey, L. E. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-oncol. 4, 39–43 (2002).
Sakajiri, S. et al. Histone deacetylase inhibitors profoundly decrease proliferation of human lymphoid cancer cell lines. Exp. Hematol. 33, 53–61 (2005).
Byrd, J. C. et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 105, 959–967 (2005).
Inoue, S. et al. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ. 11, S193–S206 (2004).
Nimmanapalli, R., Fuino, L, Stobaugh, C., Richon, V. & Bhalla, K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr–Abl-positive human acute leukemia cells. Blood 101, 3236–3239 (2003).
Batova, A. et al. The histone deacetylase inhibitor AN-9 has selective toxicity to acute leukemia and drug-resistant primary leukemia and cancer cell lines. Blood 100, 3319–3324 (2002).
Cutts, S. M., Rephael, A., Nudelman, A., Hmelnitsky, I. & Phillips, D. R. Molecular basis for the synergistic interaction of adriamycin with the formaldehyde-releasing prodrug pivaloyloxymethyl butyrate (AN-9). Cancer Res. 61, 8194–8202 (2001).
Avellino, R. et al. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood 106, 1400–1406 (2005).
Teachey, D. T. et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood 107, 1149–1155 (2006).
Pear, W. S. et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med. 183, 2283–2291 (1996).
Fekete, M. R., McBride, W. H. & Pajonk F. Anthracyclines, proteasome activity and multi-drug resistance. BMC Cancer 5, 114 (2005).
Cortes, J. et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin. Cancer Res. 10, 3371–3376 (2004).
Sutheesophon, K. et al. Histone deacetylase inhibitor depsipetide (FK228) induces apoptosis in leukemic cells by facilitating mitochondrial translocation of Bax which is enhanced by the proteasome inhibitor bortezomib. Acta Haematol. 115, 78–90 (2006).
Arguello, F. et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity in vivo against human leukemia and lymphoma xenografts. Blood 91, 2482–2490 (1998).
Almenara, J., Rosato, R. & Grant, S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA). Leukemia 16, 1331–1343 (2002).
Dasmahapatra, G., Almenara, J. A. & Grant, S. Flavopiridol and histone deacetylase inhibitors promote mitochondrial injury and cell death in human leukemia cells that overexpress Bcl-2. Mol. Pharmacol. 69, 288–298 (2006).
Rosato, R. R., Dai, Y., Almenara, J. A., Maggio, S. C. & Grant, S. Potent antileukemic interactions between flavopiridol and TRAIL/Apo2L involve flavopiridol-mediated XIAP downregulation. Leukemia 18, 1780–1788 (2004).
Yu, C., Krystal, G., Dent, P. & Grant, S. Flavopiridol potentiates STI571-induced mitochondrial damage and apoptosis in BCR–ABL-positive human leukemia cells. Clin. Cancer Res. 8, 2976–2984 (2002).
Karp, J. E. et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-β-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin. Cancer Res. 11, 8403–8412 (2005).
Banker, D. E. et al. The t(8;21) translocation is not consistently associated with high Bcl-2 expression in de novo acute myeloid leukemias of adults. Clin. Cancer Res. 4, 3051–3062 (1998).
Campos, L. et al. Expression of BCL-2 proto-oncogene in adult acute lymphoblastic leukemia. Leukemia 10, 434–438 (1996).
Hogarth, L. A. & Hall, A. G. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood 93, 2671–2678 (1999).
Tauchi, T. et al. BCL-2 antisense oligonucleotide genasense is active against imatinib-resistant BCR–ABL-positive cells. Clin. Cancer Res. 9, 4267–4273 (2003).
Marcucci, G. et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood 101, 425–432 (2003).
Gorre, M. E., Ellwood-Yen, K., Chiosis, G., Rosen, N. & Sawyers, C. L. BCR–ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR–ABL chaperone heat shock protein 90. Blood 100, 3041–3044 (2002).
Crespo, M. et al. ZAP-70 expression in normal pro/pre B cells, mature B cells and in B-cell acute lymphoblastic leukemia. Clin. Cancer Res. 12, 726–734 (2006).
Rahmani, M. et al. Cotreatment with suberanoylanilide hydroxamic acid and 17-allylamino 17-demethoxygeldanamycin synergistically induces apoptosis in Bcr–Abl+ cells sensitive and resistant to STI571 (imatinib mesylate) in association with down-regulation of Bcr–Abl, abrogation of signal transducer and activator of transcription 5 activity, and Bax conformational change. Mol. Pharmacol. 67, 1166–1176 (2005).
Pelicano, H. et al. Targeting Hsp90 by 17-AAG in leukemia cells: mechanisms for synergistic and antagonistic drug combinations with arsenic trioxide and Ara-C. Leukemia 20, 610–619 (2006).
George, P. et al. Combination of the histone deacetylase inhibitor LBH589 and the HSP90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood 105, 1768–1776 (2005).
George, P. et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 64, 3645–3652 (2004).
Hawkins, L. M., Jayanthan, A. A. & Narendran, A. Effects of 17-allylamino-17-demethoxygeldanamycin (17-AAG) on pediatric acute lymphoblastic leukemia (ALL) with respect to Bcr–Abl status and imatinib mesylate sensitivity. Pediatr. Res. 57, 430–437 (2005).
Mesa, R. A. et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood 106, 318–327 (2005).
Kastan, M. B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004). A comprehensive review of the genes and pathways involved in DNA-damage responses. Insights into the mechanisms involved in these response pathways might teach us new ways to more effectively treat leukaemia.
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Sawyers C. Targeted cancer therapy. Nature 432, 294–297 (2004).
Karagiannis, T. C. & El-Osta, A. RNA interference and potential therapeutic applications of short interfering RNAs. Cancer Gene Ther. 12, 787–795 (2005).
Thomas, M., Gessner, A., Vornlocher, H. P., Hadwiger, P., Greil, J. & Heidenreich, O. Targeting MLL–AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood 106, 3559–3566 (2005). In vitro and severe combined immune deficiency mice studies indicating that targeted inhibition of MLL–AF4 fusion gene expression with short interfering RNAs might be an effective and highly specific treatment of this therapy-resistant leukaemia.
Lang, P. et al. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood, 103, 3982–3985 (2004).
Blair, A., Goulden, N. J., Libri, N. A., Oakhill, A. & Pamphilon, D. H. Immunotherapeutic strategies in acute lymphoblastic leukaemia relapsing after stem cell transplantation. Blood Rev. 19, 289–300 (2005).
Imai, C., Iwamoto, S. & Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383 (2005).
Sparreboom, A., Baker, S. D. & Verweij, J. Paclitaxel repackaged in an albumin-stabilized nanoparticle: handy or just a dandy? J. Clin. Oncol. 23, 7765–7767 (2005).
Cheok, M. H. & Evans, W. E. Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nature Rev. Cancer 6, 117–129 (2006). A comprehensive review of the role pharmacogenomics has in improving the therapeutic index of antileukaemic agents by facilitating appropriate dose individualization.
Rocha, J. C. et al. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood 105, 4752–4758 (2005).
Cheng, Q., Yang, W., Raimondi, S. C., Pui, C. H., Relling, M. V. & Evans, W. E. Karyotypic abnormalities create discordance of germline genotype and cancer cell phenotypes. Nature Genet. 37, 878–882 (2005).
Acknowledgements
This work was supported by grants from the United States National Institutes of Health and by the American Lebanese Syrian Associated Charities.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
S.J. is a consultant for Genzyme Pharmaceuticals, Bioenvision Pharmaceuticals and Enzon Pharmaceuticals.
Related links
Glossary
- Allogeneic haematopoietic stem-cell transplantation
-
Transplantation with haematopoietic stem cells obtained from different individuals of the same species.
- Apoptosis
-
Programmed cell death.
- Aneuploidy
-
Having a chromosome number that is not the normal diploid number.
- FLT3
-
This class 3 receptor tyrosine kinase (fms-related tyrosine kinase-3) has an important role in normal haematopoiesis. Constitutive activation of the gene contributes to the abnormal growth of leukaemic cells.
- MLL
-
This gene (mixed-lineage leukaemia), located on chromosome 11, is homologous to the trithorax gene of Drosophila and displays many features of a transcription factor and of a DNA methytransferase. It fuses with AF4 (ALL fused gene from chromosome 4) to form t(4;11) translocation. The MLL–AF4 fusion protein disrupts the normal expression pattern of homeobox genes, causing a change in the self-renewal and growth of leukaemia stem cells and progenitor cells.
- NOTCH1
-
This gene — Notch homologue-1, translocation-associated (Drosophila) — encodes a member of the transmembrane protein family, which has a role in the developmental processes of various tissues. Constitutive Notch signalling in haematopoietic progenitors disrupts both normal T-cell and B-cell development and leads to T-cell ALL.
- FLAG regimen
-
A combination chemotherapy with fludarabine followed by Ara-C (cytarabine) once daily for 5 days, followed by G-CSF (granulocyte colony stimulating factor) daily for 7 days.
- Platelet-count recovery
-
Recovery of the number of platelets in the blood towards a normal count.
- Hand–foot syndrome
-
A chemotherapy-induced cutaneous reaction characterized by painful, oedematous symmetrical erythema on the palms and soles.
- Tumour-lysis syndrome
-
Lysis of a large number of malignant cells resulting in biochemical abnormalities and dysfunction of multiple organs.
- CNS relapse
-
Recurrence of leukaemia in the central nervous system (CNS).
- Extramedullary relapse
-
Recurrence of leukaemia at a site other than bone marrow, such as the central nervous system, testes, ovary, thymus or lymph node.
- Hyper CVAD chemotherapy regimen
-
A combination chemotherapy with hyperfractionated cyclophosphamide, vincristine, adriamycin (doxorubicin), and dexamethasone.
- Pancytopaenia
-
An abnormal reduction in the number of erythrocytes, leukocytes and platelets in the blood.
- ABL, KIT and PDGFR kinases
-
The ABL (Abelson) gene is located on chromosome 9 and encodes a tyrosine kinase, the role of which in normal haematopoiesis is unclear. It fuses with BCR gene on chromosome 22 to form BCR–ABL fusion gene, resulting in unregulated tyrosine kinase activity in t(9;22) Philadelphia-chromosome-positive CML or ALL. KIT gene encodes a transmembrane receptor in the PDGF (platelet-derived growth-factor receptor) family that has tyrosine kinase activity and has a role in haematopoiesis.
- SRC
-
This non-receptor sarcoma virus oncogene (SRC) is a tyrosine kinase that is involved in several cellular processes, including growth-factor and integrin-dependent signalling, cell-cycle control and regulation of apoptosis.
- T315I
-
A single mutation deep in the ATP-binding pocket of the ABL tyrosine kinase (T315I) confers a high degree of resistance to imatinib mesylate.
- Accelerated or blastic phase
-
A more symptomatic phase of chronic myeloid leukaemia, characterized by increased numbers of bone-marrow blasts and blood basophils, increased spleen size, appearance of extramedullary tumour masses and poor response to therapy. Blast crisis is the most severe manifestation of the accelerated phase.
- FHIT
-
FHIT (fragile histidine triad) is a candidate tumour suppressor gene located at chromosome 3p14.
Rights and permissions
About this article
Cite this article
Pui, CH., Jeha, S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov 6, 149–165 (2007). https://doi.org/10.1038/nrd2240
Issue Date:
DOI: https://doi.org/10.1038/nrd2240
This article is cited by
-
Fast H3K9 methylation promoted by CXCL12 contributes to nuclear changes and invasiveness of T-acute lymphoblastic leukemia cells
Oncogene (2022)
-
Panobinostat (LBH589) increase survival in adult xenografic model of acute lymphoblastic leukemia with t(4;11) but promotes antagonistic effects in combination with MTX and 6MP
Medical Oncology (2022)
-
New targets for therapy: antigen identification in adults with B-cell acute lymphoblastic leukaemia
Cancer Immunology, Immunotherapy (2020)
-
A fully human anti-IL-7Rα antibody promotes antitumor activity against T-cell acute lymphoblastic leukemia
Leukemia (2019)
-
Diagnostic value of the dual-luciferase report assay for predicting response to glucocorticoid in children with acute lymphoblastic leukemia
Clinical and Translational Oncology (2017)