Key Points
-
The mitogen-activated protein kinase (MAPK) pathway controls the growth and survival of a broad spectrum of human tumours.
-
Activating mutations in RAS and RAF result in activation of the MAPK pathway and are present in a large percentage of solid tumours.
-
The central role of RAF and MAPK kinase (MEK) in transmitting signals through the RAS–MAPK pathway make these kinases promising targets of anticancer drugs.
-
MEK inhibitors are the first highly selective inhibitors of the MAPK pathway to enter the clinic.
-
Emerging clinical data show promising hints that suppression of the MAPK pathway can be achieved without unacceptable toxicity levels.
Abstract
The RAS–mitogen activated protein kinase (MAPK) signalling pathway has long been viewed as an attractive pathway for anticancer therapies, based on its central role in regulating the growth and survival of cells from a broad spectrum of human tumours. Small-molecule inhibitors designed to target various steps of this pathway have entered clinical trials. What have we recently learned about their safety and effectiveness? Will the MAPK pathway prove amenable to therapeutic intervention?
Similar content being viewed by others
Main
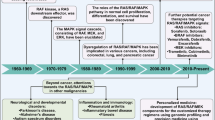
A diverse array of growth factors, cytokines and proto-oncogenes transduce their growth-promoting signals through the activation of the small G protein RAS, which leads to activation of the serine/threonine kinase RAF, and then to activation of the mitogen-activated protein kinase (MAPK) kinase (MEK). MEK then phosphorylates and activates MAPK, also known as extracellular signal-regulated kinase (ERK). Dating back to the early 1990s, elucidation of the various protein interactions and phosphorylation events that mediate this pathway has made it one of the best-characterized growth-factor-mediated signalling pathways.
Anchorage-independent cell-cycle progression is one hallmark of the neoplastic phenotype1,2. The various growth factors and cell-adhesion receptors that are involved in regulating anchorage-dependent cell-cycle entry stimulate a myriad of signalling pathways, including the phosphatidylinositol 3-kinase (PI3K) and MEK–ERK cascades (Fig. 1). In addition, these pathways regulate other responses associated with cell adhesion such as anoikis (apoptosis that occurs when cells detach from their substrate) and cell motility (Fig. 2). These events are mediated by both transient reversible steps as well as by changes in gene expression. Comprehensive reviews of these regulatory processes have been published elsewhere3,4. We will discuss what we have recently learned about the importance of the RAS–MEK–ERK pathway in tumour growth and progression from studies involving small-molecule inhibitors of one or more components of this cascade. Recent data from clinical trials with these inhibitors have provided important new information about the toxicity and efficacy of targeting the MAPK pathway.
Following ligand binding, growth-factor receptor tyrosine kinases such as the platelet-derived growth factor receptor (PDGFR), the vascular endothelial growth factor receptor (VEGFR), the receptor ERBB, and the fibroblast growth factor receptor (FGFR) become activated. This induces the binding of adaptor proteins such as growth-factor-receptor-bound protein 2 (GRB2) that bind to specific phosphorylated residues on the cytoplasmic tails of activated receptors. In cooperation with GRB2, the guanine-nucleotide exchange factor SOS activates RAS, by catalysing the replacement of GDP with GTP. In its GTP-bound form, RAS activates the kinase activity of RAF and its downstream signalling cascade. RAF phosphorylates the mitogen-activated protein kinase (MAPK) kinase (MEK), which phosphorylates and activates MAPK/extracellular signal-regulated kinase (ERK). There exist several negative regulators of this pathway. These regulatory molecules include RAF kinase inhibitor protein (RKIP), which interferes with MEK phosphorylation81; RAS and RAB interactor 1 (RIN1), which competes with RAF for binding to activated RAS82; IMP (impedes mitogenic signal propagation), which interacts with kinase suppressor of RAS (KSR) to prevent recruitment of MEK to activated RAF83; and the MKP (MKP1,3) family of MAPK phosphatases, which reduce the activation state of the RAF–MEK–ERK module84,85. Other negative regulators include both AKT and serum/glucocorticoid inducible kinase (SGK), which can phosphorylate BRAF86,87. Positive regulators of RAF activity, such as protein kinase C (PKC), SRC, p21-activated kinase (PAK) and 14-3-3, activate RAF in a RAS-independent manner. This pathway can also become activated by adhesion of integrins to specific extracellular-matrix molecules. These interactions activate focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3K). PI3K is also activated by RAS. Stimulation of this pathway leads to activation of the GTP-binding protein RAC, which interacts with the NCK–PAK complex, thereby leading to PAK, and subsequently RAF, activation.
Once the extracellular signal-regulated kinases ERK1 and ERK2 are activated through the RAS–RAF–MEK–MAPK signalling pathway shown in Fig. 1, they mediate a range of cellular effects. They activate several subsequent kinases, including mitogen-activated protein kinase (MAPK)-interacting kinases (MNK1 and MNK2), mitogen- and stress-activated kinases (MSK1 and MSK2), and 90-kDa ribosomal S6 protein kinase (RSK). They also activate transcription factors such as the peroxisome-proliferator-activated receptor-γ (PPARγ), ELK1, ETS, signal transducer and activator of transcription 1 (STAT1) and STAT3. The resulting changes in gene regulation affect cell-cycle progression and/or cell motility in a cell-dependent manner. MEK, MAPK kinase.
Cell-cycle regulation
Cell proliferation can be activated by activation of growth-factor receptors and/or binding of integrins to specific extracellular-matrix molecules during cell adhesion. Activation of these receptors is associated with the induction of cyclin D1 and downregulation of endogenous cyclin-dependent kinase (CDK) inhibitors such as p21 (also known as WAF1 or CIP1) and p27 (also known as KIP1) (Refs 5,6). Early studies showing a link between the MEK–ERK pathway and cell-cycle machinery included the demonstration that blockade of thrombin-induced proliferation of Chinese hamster lung fibroblasts correlated with suppression of ERK activation7. Similarly, dominant-negative forms of ERK, as well as ERK antisense nucleotides, inhibited proliferation of NIH3T3 fibroblasts8. In addition, studies using pharmacological inhibitors of MEK9,10 or activated MAPK phosphatase 1 (MKP1), a negative regulator of MEK11, significantly altered the ability of these cells to proliferate in response to growth-factor stimulation.
The expression of cyclin D1 was ultimately shown, through the expression of dominant-negative or activated forms of MEK, to be controlled by MEK–ERK signalling12,13. This induction is thought to be mediated, in part, by activation of the AP-1 transcription factor14. Although data indicate that ERK activation is also required for cyclin D1 expression, the intensity and duration of the signalling through this pathway ultimately determines whether a cell undergoes differentiation, proliferation or cell-cycle arrest15.
Different RAF isoforms, for example, fine tune the cell-cycle regulatory machinery16. In response to a strong RAF signal, a G1-specific cell-cycle arrest, through induction of p21, leads to inhibition of cyclin-D- and cyclin-E-dependent kinases17. This finding is consistent with a report that sustained activation of MEK correlates with cell-cycle arrest18. However, moderate RAF activity is sufficient to induce cyclin D expression and DNA synthesis17. Negative regulation of RAF is mediated by a range of proteins, such as AKT and serum/glucocorticoid inducible kinase19,20. Furthermore, the phosphorylation status and expression levels of a range of early gene products of the MAPK signalling pathway, such as of the transcription factors FOS, MYC and JUN, have also been shown to dictate biological outcome21. FOS has been shown to function as a sensor for ERK signal duration. When ERK activation is transient, its activity declines before FOS protein accumulates. By contrast, sustained ERK signalling results in phosphorylation of FOS, leading to its stabilization, thereby positively impacting cell-cycle entry22.
How does one integrate the observation that high levels of RAF activation can result in cell-cycle arrest with the well-established role of MEK–ERK signalling in promoting G1 progression? An explanation is provided by the finding that CDK-inhibitor family members can positively regulate the activation of cyclin-D-dependent kinases23. This observation is consistent with the induction of p21 in growth-factor-stimulated fibroblasts occurring in the early part of the cell cycle followed by its decline at mid G1 (Ref. 24). This pattern resembles the activation of ERKs during the G1 to G1/S transition25.
Given the role of the MEK–ERK signalling pathway in mediating both cell-cycle progression and arrest, it is surprising that most tumours have increased, sustained activation of the RAF–MEK–ERK pathway. One possible explanation could be that this pathway also contributes to one of the mechanisms that tumour cells use to promote their own survival, such as by controlling induction of apoptosis.
Survival signalling
Studies in several experimental model systems have provided insight into the involvement of RAF–MEK–ERK signalling in control of cell survival. As reviewed elsewhere, members of the RAF family (BRAF and c-RAF/RAF1) have been described as important mediators of anti-apoptotic activity26. Although the anti-apoptotic activity of the RAF family seems to be established, the molecular steps are just beginning to be defined. Both the kinase activity and kinase-independent mechanisms of RAF are believed to mediate its anti-apoptotic functions. Independent of its ability to stimulate MEK–ERK signalling, RAF is believed to directly antagonize the death-promoting activity of apoptosis signal-regulating kinase 1 through a physical interaction between the two proteins27.
The classical pathway by which RAF regulates apoptosis involves its kinase activity, which regulates both the transcriptional activity of the cell as well as the activity of pro-apoptotic proteins. Functionally, the signalling pathways can be divided between MEK-dependent and -independent events. In a MEK-dependent manner, activation of ERK and subsequently of the 90-kDa ribosomal S6 protein kinase (RSK, Fig. 2) leads to the phosphorylation and inactivation of the pro-apoptotic protein BAD28. RSK activation also leads to phosphorylation of the transcription factor CREB, thereby promoting survival29. However, studies showing that RAF-mediated activation of the pro-survival transcription factor NF-κB occurs in the absence of ERK activation indicates the existence of a MEK-independent mechanism in RAF's repertoire of anti-apoptotic functions30. Data obtained from RAF1-null mice, as well as those obtained from studies using specific MEK inhibitors or activated and dominant-negative forms of MEK, support the occurrence of both MEK-independent and MEK-dependent anti-apoptotic mechanisms31,32.
Targeting the MAPK signalling pathway
As the MAPK signalling pathway not only promotes cell proliferation, but also mediates cell survival and is upregulated in cancer cells, it seems to be a good therapeutic target. Several inhibitors of MAPK signalling have therefore been developed. What are the effects of inhibiting this pathway in animal models? Erk1−/− mice are fertile and have no apparent abnormalities. However, they do have a twofold reduction in the number of mature thymocytes. Furthermore, T-cell proliferation is reduced in response to activating factors33. The involvement of the MAPK pathway in immune signalling has therefore raised valid concerns about the potential toxicity of inhibitors directed against this pathway. In addition, Mek1−/− mice undergo embryonic lethality, resulting from impaired placental development. Subsequent demonstration that Mek1−/− fibroblasts were defective in fibronectin-mediated cell migration, despite the fact that MEK2 and ERK activation were normal, indicated that MEK1 might have additional roles other than activation of ERK34. If so, blockade of this pathway could be toxic.
There has also been widespread concern that effective blockade of the MAPK pathway would prove too toxic to normal proliferating cells, such as those in the gastrointestinal tract and skin. Might it be possible to develop small-molecule inhibitors that have sufficient potency and selectivity to inhibit this signalling pathway specifically in cancer cells?
Approaches to therapeutic intervention
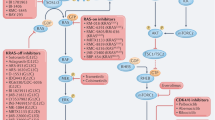
The regulation of a large number of cellular processes depends on the activation state of ERK, so blocking the RAS–MAPK pathway conceivably has use against a multitude of human diseases and conditions including cancer35,36,37, inflammatory diseases38,39, pain40 and cardiac hypertrophy41,42. Because of widespread concerns regarding the potential toxicity of MAPK-pathway inhibitors, efforts in this area have largely been expended in the oncology arena, because of the life-threatening nature of the disease. Clearly, there exists a strong rationale for exploring the potential of MAPK-pathway inhibitors as anticancer agents, in part based on the broad spectrum of human tumours known to show activated signalling through this pathway (Table 1). Three proteins have received the most attention as targets for pharmacological intervention of the MAPK pathway: RAS, RAF and MEK. Each of these proteins has unique features that make it a good therapeutic target, from a scientific rationale. What are their relative merits and what has been learned from the study of various small-molecule inhibitors, in both the preclinical and clinical arenas?
Farnesyltransferase inhibitors. Extensive effort has been expended from numerous academic and pharmaceutical laboratories to investigate the therapeutic potential of inhibiting RAS activity with selective farnesyltransferase inhibitors (FTIs). RAS, which does not have a transmembrane domain, must have an ISOPRENOID group attached to it, through a process known as prenylation, to facilitate its localization to the plasma membrane. By inhibiting the post-translational addition of this farnesyl group to RAS by farnesyltransferase, it was thought that FTIs would be able to target a broad range of human tumours in which RAS was constitutively activated. The rationale for this approach has been discussed in greater detail elsewhere43,44.
Preclinical evaluation of numerous small-molecule FTIs, covering chemically diverse structural classes, yielded promising reports of activity. Anticancer effects included antiproliferative, pro-apoptotic and anti-angiogenic activities45. However, concerns about this approach began surfacing when investigators learned that higher concentrations of FTIs were required to inhibit oncogenic KRAS compared with wild-type RAS or oncogenic HRAS46. It was subsequently shown in preclinical models that tumours that express KRAS — the most commonly detected form of activated RAS — escape FTI inhibition.
The ability of several FTIs to treat a broad range of human cancers has nonetheless been tested in clinical trials. These agents, including BMS-214662, L-778123, SCH-66336 (Sarasar), R115777 (Zarnestra) and AZD3409, represent structurally distinct small molecules. For a comprehensive review of the clinical testing status of these agents, see Ref. 45. The most extensive clinical evaluation has been carried out with Zarnestra, which has been studied in patients diagnosed with a range of cancers, encompassing acute and chronic leukaemias, multiple myeloma, non-small-cell lung cancer, and breast, prostate, colorectal and pancreatic cancers. Clinical results with R115777 have largely been disappointing (Table 2). The most notable effects were observed when this drug was administered as a single agent to patients with haematopoietic malignancies. The lack of objective responses in a Phase III clinical trial of patients with advanced pancreatic cancer47, where the incidence of KRAS mutations is extremely high, provides further evidence that FTIs are not successful in treating tumours that are associated with RAS activation.
There is little doubt, however, that these drugs actually inhibit farnesyltransferase activity in patients. Translational studies with the FTIs R115777 and SCH66336 have shown significant inhibition of prenylated proteins, namely the chaperone protein HDJ2 and the intranuclear intermediate-filament protein prelamin A. FTI research is now focused on the search for farnesylated proteins that are required for disease pathogenesis and that are strongly inhibited by these drugs. Candidate proteins include centromere-associated proteins, the PRL family of phosphatases, the RAS-related protein RHEB, and the Rho family member RND44. Alternatively, the real target(s) of FTI-mediated efficacy might not be farnesylated. It is known for instance that RHOB is not only farnesylated, but is also a substrate for geranylgeranyltransferase — another prenylation enzyme, which adds a 20-carbon, rather than a 15-carbon, lipid moiety to signalling proteins. A gain-of-function role for geranylgeranylated RHOB has been described48. Although there could be a specific therapeutic niche in which FTIs are most effective, clinical data obtained so far leads us to conclude that these agents do not represent a viable approach to blocking signal transduction through the RAS–MAPK pathway.
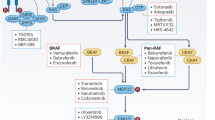
RAF inhibitors. The RAF family — ARAF, BRAF and RAF1 — are at the centre of a highly regulated control module involving both negative and positive regulatory interactions with a large array of signalling molecules. RAF phosphorylates the downstream kinase MEK on two serine residues to activate it. Initially, interest in inhibitors of the kinase activity of RAF came about as a consequence of the finding that RAF was an important downstream effector of RAS49. It was therefore reasonable to speculate that inhibition of this kinase might afford a broader spectrum of antitumour activity than agents directed against a single upstream growth-factor receptor, such as members of the ERBB or vascular endothelial growth factor (VEGF) receptor families. The discovery of oncogenic BRAF mutations in human tumours has fuelled further interest in exploring the anticancer potential of inhibitors that block the kinase activity of RAF50.
BRAF mutations are most prevalent in melanomas, occurring in nearly 70% of tumour samples examined50,51, but are also found at a significant frequency in other cancers, including cancers of colorectal50,52,53, ovarian54 and thyroid origin55,56. Interestingly, roughly 90% of the activating mutations involve the replacement of Val599 with a glutamate residue. This site lies within the kinase activation loop, resulting in constitutive activation of BRAF. BRAF activity is enhanced by this mutational change presumably because its negative charge effectively mimics phosphorylation of the activation loop.
The functional consequences of knocking down BRAF activity in melanoma with small interfering RNA has confirmed that its oncogenic activity mediates ERK signalling, induction of proliferation and protection from apoptosis57,58. These studies have provided further impetus in exploiting BRAF for pharmacological intervention in patients with cancer. As reviewed by Bollag et al., several small-molecule RAF inhibitors have now been reported59. According to published reports, only one of these, BAY 43-9006, has reached the clinical testing stage. BAY 43-9006 was discovered through a combinatorial chemistry approach aimed at studying structural modifications to a 3-thienyl urea chemical series60. Subsequently, this bis-aryl, which inhibits recombinant RAF with an IC50 of 12 nM, was shown to have in vivo anti-tumour activity against colon, pancreatic and ovarian xenograft models60.
BAY 43-9006 is now undergoing clinical evaluation, with several Phase I and Phase II trials now completed (Table 2). It has been reported that this agent is generally well tolerated, with the most common toxicities involving the gastrointestinal tract (diarrhoea) and the skin (rash). These side effects seem consistent with those expected from a MAPK-pathway inhibitor, which would be predicted to affect tissues known to undergo rapid turnover, like the gut and skin. Although clinical trials have revealed substantial interpatient variability in the pharmacokinetics of BAY 43-9006, dose-dependent increases in exposure were consistently observed61. A total of three Phase I trials were carried out, in which the incidence of stable disease ranged from 33–43%. Furthermore, partial responses were observed for two patients, one with renal-cell cancer and one with hepatacellular carcinoma62. Whereas Phase II trials were carried out in patients with several different tumour types, including melanoma, as well as renal-cell, colorectal and hepatocellular carcinomas, Phase III trials seem to be focusing on patients with renal-cell carcinoma. Will RAF prove to be a valid anticancer drug target? There are several studies indicating that this question can not be addressed by BAY 43-9006, because of its lack of specificity. It was earlier reported that BAY 43-9006 inhibits RAF1 without affecting other protein kinases60. Recent studies, however, have reported that this agent inhibits a range of other kinases, including many that are known to regulate proliferation and survival of tumour cells, such as platelet-derived growth factor receptor (PDGFR) and kinase insert domain receptor (KDR, also known as VEGFR2) tyrosine kinases109. So, although BAY 43-9006 has promise as an anticancer agent, based on reports of its clinical activity, it is difficult to assess the impact of RAF inhibition on observed clinical outcomes.
Clinical activity of BAY 43-9006 against renal-cell carcinoma could very likely be a consequence of its effective inhibition of the VEGF receptor KDR. This supposition is based on a report that bevacizumab and SU11248, which are distinctly different types of VEGFR inhibitors, both have antitumour activity against human renal cancers. The known susceptibility of renal tumours to VEGFR inhibitors, along with recent reports on VEGFR inhibition by BAY 43-9006, make it tempting to speculate that VEGFR is this drugs true antitumour target, rather than RAF63. Clinical samples from patients treated with BAY 43-9006 could also be examined to determine if MAPK signalling is reduced, and to determine the relative contribution of RAF inhibition in patient response. There have been encouraging reports that this drug inhibits phorbol-ester-stimulated ERK levels in patients' peripheral-blood lymphocytes, so this could be a useful biomarker64. Further studies should include a similar biomarker analysis of human tumour biopsies.
Ongoing efforts in this area are no doubt being directed towards the design and development of BRAF-specific kinase inhibitors, as in human cancers, oncogenic mutations have only been identified in the BRAF isoform. RNA interference studies have successfully demonstrated that depletion of BRAF, but not ARAF or RAF1, in melanoma cell lines blocks ERK activity57. It is not clear, however, whether BAY 43-9006 has effects on these other isoforms, as the RAF homologue kinase domains are relatively highly conserved — there is 78% and 81% identity between BRAF compared with ARAF and RAF1, respectively. The observation that activating mutations are always found in BRAF might be due to differences in the number of RAS-GTP-dependent phosphorylation sites required for maximal activation — two versus four sites in the case of BRAF and RAF1, respectively65,66.
A key breakthrough in our understanding of how BRAF mutations lead to transformation was provided by the recent elucidation of the crystal structure of this enzyme67. Richard Marais and his colleagues successfully solved the structure of isolated wild-type and mutant BRAF kinase domains (Box 1) bound to the inhibitor BAY 43-9006. The binding pattern of this inhibitor shows that the hinge region and the conserved Asp–Phe–Gly motif present in the activation loop (kinase subdomain VIII) are important for the interaction between this inhibitor with the enzyme. Information provided by these crystal-structure analyses also explained why the ATP-binding site and the activation loop are preferential targets for mutational events. Interestingly, oncogenic mutations affecting the equivalent position to Val599 in BRAF are known to occur in various tyrosine kinases, including PDGFR kinase family members such as KIT68. Structural studies are proving to be invaluable for addressing the reasons that such different kinases have oncogenic hot spots in common. In the meantime, this information can also be exploited in the design of cancer drugs tailored to these molecular targets.
MEK inhibitors. The rationale for targeting MEK is similar to that of targeting RAF — because it has a central role in the MAPK pathway and in transducing proliferative stimuli in a broad spectrum of human cancer cells. In vitro expression of constitutively active forms of MEK has been shown to result in transformation, giving rise to highly tumorigenic cell lines69,70. Similarly, MEK has been implicated in the development of a broad range of human tumours71. Studies carried out in a large number of tumour types have indicated the importance of MEK, which activates ERK, in driving their proliferation and, often, progression (Table 1).
MEK proteins are dual-specificity kinases that contain two consensus kinase motifs, one involved in phosphorylation of serine/threonine residues and another in phosphorylation of tyrosine residues. Two MEK homologues, MEK1 and MEK2 are ubiquitously expressed in mammals. Both of these MEKs sequentially phosphorylate ERK1 and ERK2 at two sites — Tyr185 followed by Thr183 (Ref. 72). For a discussion of the biological attributes that delineate key differences between MEK1 and MEK2, see Ref. 73. Their high degree of sequence homology makes it likely that a small-molecule MEK inhibitor would target both homologues.
The development of pharmacological inhibitors of MEK was launched with the discovery of PD098059 (Ref. 9). Because of its pharmaceutical limitations, this compound was made available to the academic community and subsequently became an invaluable tool for exploring the role of the MAPK pathway in an array of physiological processes. U0126, a MEK inhibitor shown to be more potent than PD98059, has also become a widely used reagent for investigating the role of this pathway in cellular events74. The first MEK inhibitor reported to inhibit tumour growth in vivo was PD184352 (subsequently named CI-1040)75. This compound, which was shown to be orally active in early studies, was shown to significantly inhibit the growth of colon carcinomas in animal models. Importantly, efficacy was achieved at well-tolerated doses and correlated with a reduction in the levels of activated ERK in tumour samples. Based on a promising preclinical profile that encompassed effects on proliferation, survival, invasion and secretion of pro-angiogenic growth factors, this agent was advanced into clinical oncology trials75,76.
CI-1040 has been evaluated in Phase I and Phase II trials76,77. Because of the poor metabolic stability and bioavailability of this agent observed in Phase I trials, high doses were administered to patients in Phase II trials (Table 2). Whereas there was encouraging evidence of antitumour activity in patients in the Phase I trial, similar results were not observed in Phase II trials, so development of this agent was terminated. Nonetheless, the finding that levels of phosphorylated ERK were reduced by roughly 50% or greater in all of the evaluable tumour biopsies taken during the Phase I trial indicated that this drug did affect its target in humans, and that it might be possible to improve the pharmaceutical properties of CI-1040. As CI-1040 was clinically well tolerated at doses that clearly resulted in significant MEK inhibition, would it be possible to develop more potent MEK-targeted agents with greater systemic exposure?
PD0325901, which is structurally highly similar to CI-1040, was subsequently developed as a significantly more potent MEK inhibitor. It has an IC50 of 1 nM against activated MEK1 and MEK2 (J.S.S.-L., unpublished observations). This compound, which has subnanomolar IC50 values in cells, is also significantly more potent than CI-1040 in vivo. Anticancer activity of PD 0325901 has been demonstrated for a broad spectrum of human tumour xenografts, significantly inhibiting the growth of six out of seven human tumour models tested. The improved anticancer activity of PD0325901 over CI-1040 is probably due to several contributing pharmacological factors, including longer duration and greater potency of MEK inhibition, as well as greater solubility leading to improved bioavailability, and increased metabolic stability. Phase I trials with this agent are now underway and are focusing on patients with solid tumours who are expected to show activation of the MAPK pathway.
The benzimidazole ARRY-142886 has also been reported to be a highly potent MEK inhibitor, with an IC50 of 12 nM against purified MEK110. This compound was reported to inhibit basal phosphorylation of ERK in a range of human tumour cell lines, with an IC50 in the 10 nM range. ARRY-142886 has also been reported to be efficacious against a panel of tumour xenografts, encompassing tumours of pancreatic, colon, breast, lung and skin origin. Based on its collective preclinical profile, ARRY-142886 was advanced into full development and recently entered clinical trials.
All three of these recently developed MEK inhibitors, CI-1040, PD0325901 and ARRY-142886, are highly selective for MEK, as indicated by their inability to inhibit a multitude of serine/threonine and tyrosine kinases. None of these inhibitors compete with ATP binding. Why are these inhibitors so selective for MEK? The answer to this question was provided through structural analysis of human MEK1 or MEK2, each determined as a ternary complex of enzyme, PD0325901-like inhibitor and MgATP78 (Box 2). These studies showed that NON-COMPETITIVE INHIBITORS such as PD0325901 can bind to MEK without perturbing the ATP site. The concurrent binding of MgATP and these inhibitors does, however, cause several conformational changes in MEK1 and MEK2. These changes are thought to contribute to their unique non-competitive mechanism of inhibition and help explain the remarkable selectivity of these drugs, as this binding pocket is in a region without sequence homology to other kinases.
Validation of inhibitors of the MAPK pathway as anticancer therapeutics has been long awaited. This quest has been further hampered by the lack of suitable drug candidates to provide clear answers. The high degree of specificity of PD0325901 and ARRY-142886, combined with their favourable pharmaceutical profiles, make these compounds ideal clinical candidates for fulfilling this need.
Clinical challenges
In many ways, the challenges that we face in the design of clinical trials of inhibitors of the RAS–MAPK pathway do not differ substantially from those faced with other anticancer agents. Whether testing a signal-transduction inhibitor or even a cytotoxic agent, one does not know at the outset which tumour types will be most sensitive. However, unlike the case for cytotoxic agents, patients who benefit from treatment with a signal-transduction inhibitor are likely to share common molecular alterations of the target or pathway, such as RAS or RAF mutations driving activation of the MAPK pathway. This means that a subset of patients with certain tumour genotypes will respond to a certain drug in a more consistent manner than will an entire patient population of the same histological tumour type. Designing clinical trials that accrue patients based on the molecular profile of the tumour is therefore a special challenge. However, advances in our understanding of the molecular events that trigger oncogenic transformation make certain tumour subpopulations particularly attractive targets for a given agent. Knowing for instance that melanomas and pancreatic cancers have a high incidence of BRAF- and KRAS-activating mutations, respectively, make patients with these tumour types strong candidates for inclusion in clinical trials with MAPK-pathway inhibitors.
Are trial sponsors ready to take this leap of faith based on strong preclinical science? Only time will tell — clinical trials have often been directed towards the largest patient populations, including patients with lung, breast and colon cancers. The time has clearly arrived to believe in our targeted agents and to test in populations in which we feel that they have a good chance of being most effective, including the smaller patient populations that are enriched for a specific molecular defect. Otherwise, we face a real risk of shelving viable anticancer drugs for the wrong reasons. Along these same lines, it will be important to retrospectively profile tumour samples taken from patients, subsequently correlating the presence of various molecular markers with response outcome. By amassing information on genetic and epigenetic markers, such as RAS and RAF mutational status, commonalities in response determinants will hopefully be deduced. Although analysis of tumour samples for specific biomarkers is important for determining the in vivo effects of signal-transduction inhibitors, we will continue to face the technical challenges of securing sufficient biopsy material to perform these types of studies. Archived tumour material obtained at the time of surgery will probably serve as an invaluable resource when fresh biopsy material is not available.
Will inhibitors of the MAPK pathway be effective as single agents? As for all potential anticancer drugs, this is a formidable challenge, as a high percentage of human tumours possess more than one genetic defect. So it is likely that the ultimate niche for a MAPK-pathway inhibitor will be in combination with another signal-transduction inhibitor. For example, tumours that have upregulated signalling through the RAS–MAPK pathway also frequently have high levels of activated AKT, which is activated by PI3K. The overall result alters the balance between survival versus apoptosis signaling, resulting in cell survival54. For maximal therapeutic benefit, it might be necessary to combine agents directed at blocking both pathways. As AKT and PI3K inhibitors, or other inhibitors of this pathway become available for clinical testing, their combination with MAPK-pathway inhibitors should be a high priority. In the meantime, a multitude of other targeted agents have become available that should also be tested in combination with MAPK-pathway inhibitors.
Will toxicity of RAF and MEK inhibitors preclude their ability to sufficiently shut down signalling through the MAPK pathway in tumour cells? Preclinical studies have shown that complete and continuous suppression of MAPK activation is required for maximal therapeutic effects (J.S.S.-L., unpublished observations). Ongoing studies with PD0325901 and ARRY-142866 will determine exactly how much target suppression can be tolerated in patients before unacceptable levels of damage to the gastrointestinal tract and skin occurs.
It is too early to tell whether clinical resistance to MAPK-pathway inhibitors, as has been the case with imatinib, will be encountered. A molecular explanation for acquired resistance to imatinib has been provided by detailed studies characterizing its target, the BCR–ABL chromosome-translocation product. This fusion protein undergoes mutations in its kinase domain that change Thr315 to an isoleucine residue79. This hot spot in the ATP-binding site has been also identified in other kinases, such as epidermal growth factor receptor and PDGFR, and might therefore undergo mutations that confer resistance to other drugs that target tyrosine kinases. Indeed, a patient with a gastrointestinal stromal tumour that developed resistance to imatinib was found to contain an analogous mutation in KIT80. As we know that the BRAF kinase domain also contains hot spots for oncogenic mutations, RAF inhibitors could well be subject to the same mechanisms of resistance.It has, however, been reported that BAY 43-9006 interacts with an inactive conformation of BRAF, so oncogenic mutants of BRAF might be less sensitive to a RAF-kinase inhibitor, when compared with wild-type BRAF67. It is tempting to speculate that the non-ATP-competitive inhibitors of MEK that are now in clinical trials will not be subject to this type of resistance. The very absence of activating mutations, which rendered MEK an undesirable drug target to many researchers years ago, could ultimately allow this enzyme to be an effective therapeutic target.
Future directions
There continues to be a high level of interest in targeting the RAS–MAPK pathway for the development of improved cancer therapies. So, how far have we come? MEK inhibitors represent the only 'clean', or highly selective, MAPK-pathway inhibitors now available. With clinical trial data pending, the MEK inhibitors PD0325901 and ARRY-142886 are positioned to be the first selective inhibitors of the RAS–MAPK pathway to to be validated as effective cancer therapies. However, this does not mean that RAF is no longer an important target. It has simply not been validated as such, because of the non-selectivity issue surrounding BAY 43-9006. Interest in targeting the RAS–MAPK pathway has not waned, in part due to the discovery of BRAF-activating mutations in human tumours. Many research programmes are now focused on the search for selective BRAF inhibitors. As these agents emerge, it will be of great interest to examine their pharmacological properties in the context of those observed for MEK inhibitors. As RAF is known to be involved in MEK-independent survival signalling, it is conceivable that selective BRAF inhibitors could have broader-spectrum anticancer activity than MEK inhibitors. By the same token, they could concurrently possess a higher toxicity potential.
Clearly, RAF and MEK are the only components of the RAS–MAPK pathway that are currently receiving attention as therapeutic targets. However, a multitude of other potential targets in this pathway are available for therapeutic exploration (Fig. 1). For example, a search for inhibitors of downstream targets from ERK, such as RSK, as well as positive allosteric agonists of phosphatases, such as MKP1, represent a largely unexplored area. Although the patent literature cites many examples of ERK inhibitors, none have been evaluated in clinical trials. Of all of the members of the MAPK cascade, it is tempting to speculate that SOS represents one of the most attractive antitumour targets from a functional standpoint. By disrupting the RAS–SOS interaction, one might expect that signalling through both the MAPK and PI3K pathways would be significantly impaired, thereby inhibiting both proliferation and survival of tumour cells. Unfortunately, antagonists of protein–protein interactions face high pharmaceutical hurdles, as they are difficult to develop. Overcoming these hurdles could have huge therapeutic impact.
As we look forward, we need smart drugs and smart clinical trials that take into account known genetic defects of tumours in a given patient population. In this way, we will maximize our chances of delivering improved therapies to patients in desperate need of our success.
References
Hahn, W. C. & Weinberg, R. A. Modelling the molecular circuitry of cancer. Nature Rev. Cancer 411, 331–341 (2002).
Evan, G. I. & Vousden, K. H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Grossmann, J. Molecular mechanisms of 'detachment-induced apoptosis – anoikis'. Apoptosis 7, 247–260 (2002).
Nelson, W. J. & Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 (2004).
Assoian, R. K. Anchorage-dependent cell cycle progression. J. Cell Biol. 136, 1–4 (1997).
Roovers, K. & Assoian, R. K. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22, 818–826 (2000).
Meloche, S., Seuwen, K., Pages, G. & Pouyssegur, J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol. Endocrinol. 6, 845–854 (1992).
Pages, G. et al. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl Acad. Sci. USA 90, 8319–8323 (1993).
Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J. & Saltiel, A. R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA 92, 7686–7689 (1995). The first report of a small-molecule MEK inhibitor, PD098059. Findings with this compound have subsequently been reported in over 2,000 publications, documenting the central role of the MAPK pathway in a diverse array of physiological events, including tumour proliferation, differentiation, angiogenesis and survival.
Alessi, D. R., Cuenda, A., Cohen, P., Dudley, D. T. & Saltiel, A. R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J. Biol. Chem. 270, 27489–27494 (1995).
Brondello, J. M., McKenzie, R. R., Sun, H., Tonks, N. K. & Pouyssegur, J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene 10, 1895–1904 (1995).
Lavoie, J. N., L'Allemain, G., Brunet, A., Muller, R. & Pouyssegur, J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271, 20608–20616 (1996).
Cheng, M., Sexl, V., Sherr, C. J. & Roussel, M. Assembly of cyclin D-dependent kinase and titration of p27kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl Acad. Sci. USA 95, 1091–1096 (1998).
Cook, S. J., Aziz, N. & McMahon, M. The repertoire of Fos and Jun proteins expressed during the G1 phase of the cell cycle is determined by the duration of mitogen-activated protein kinase activation. Mol. Cell. Biol. 19, 330–341 (1999).
Marshall, C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Raabe, T. & Rapp, U. R. Ras signaling: PP2A puts Ksr and Raf in the right place. Curr. Biol. 13, R635–R637 (2003).
Sewing, A., Wiseman, B., Lloyd, A. C. & Land, H. A high-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol. Cell. Biol. 17, 5588–5597 (1997).
Pumiglia, K. M. & Decker, S. J. Cell cycle arrest mediated by the MEK/migogen-activated protein kinase pathway. Proc. Natl Acad. Sci. USA 94, 448–452 (1997).
Zhang, B. H. et al. Serum- and glucocorticoid-inducible kinase SGK phosphorylate and negatively regulates B-Raf. J. Biol. Chem. 276, 31620–31626 (2001).
Guan, K. L. et al. Negative regulation of the serine/threonine kinase B-raf by Akt. J. Biol. Chem. 275, 27354–27359 (2000).
Murphy, L. O., MacKeigan, J. P. & Blenis, J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol. Cell. Biol. 24, 144–153 (2004).
Murphy, L. O., Smith, S., Chen, R. H., Fingar, D. C. & Blenis, J. Molecular interpretation of ERK signal duration by immediate early gene products. Nature Cell Biol. 4, 556–564 (2002).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Bottazzi, M. E., Zhu, X., Bohmer, R. M. & Assoian, R. K. Regulation of p21cip1 expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Cell Biol. 146, 1255–1264 (1999).
Meloche, S. Cell cycle reentry of mammalian fibroblasts is accompanied by the sustained activation of p44mapk and p42mapk isoforms in the G1 phase and their inactivation at the G1/S transition. J. Cell Physiol. 163, 577–588 (1995).
Herrera, R. & Sebolt-Leopold, J. S. Unraveling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends Mol. Medicine 8, S27–S31 (2002).
Chen, J. et al. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK–ERK independent mechanism. Proc. Natl Acad. Sci. USA 98, 7783–7788 (2001).
Shimamura, A. et al. Rsk 1 mediates a MEK–MAP kinase cell survival signal. Curr. Biol. 10, 127–135 (2000).
Bonni, A. et al. Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Baumann, B. et al. Raf induces NF-κB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc. Natl Acad. Sci. USA 97, 4615–4620 (2000).
Murakami, M. S. & Morrison, D. K. Raf-1 without MEK? Sci. STKE 99, PE30 (2001).
Ballif, B. A. & Blenis, J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)–MAPK cell survival signals. Cell Growth Differ. 12, 397–408 (2001).
Pages, G. et al. Defective thymocyte maturation in p44 MAP kinase (Erk1) knockout mice. Science 286, 1374–1377 (1999).
Giroux, S. et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9, 369–376 (1999).
Howe, A. K., Aplin, A. E. & Juliano, R. L. Anchorage-dependent ERK signaling – mechanisms and consequences. Curr. Opin. Genet. Dev. 12, 30–35 (2002).
Wada, T. & Penninger, J. M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 (2004).
Platanias, L. C. MAP kinase signaling pathways and hematologic malignancies. Blood 101, 4667–4679 (2003).
Stanton, L. A., Underhill, T. M. & Beier, F. MAP kinases in chondrocyte differentiation. Dev. Biol. 263, 165–175 (2003).
Hofman, P. Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Curr. Drug Targets. Inflamm. Allergy 3, 1–9 (2004).
Ji, R. R. Mitogen-activated protein kinases as potential targets for pain killers. Curr. Opin. Investig. Drugs 5, 71–75 (2004).
Sugden, P. H. Signalling pathways in cardiac myocyte hypertrophy. Ann. Med. 33, 611–622 (2001).
Barron, A. J., Finn, S. G. & Fuller, S. J. Chronic activation of extracellular-signal-regulated protein kinases by phenylephrine is required to elicit a hypertrophic response in cardiac myocytes. Biochem. J. 371, 71–79 (2003).
Downward, J. Targeting Ras signaling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Sebti, S. M. & Der, C. J. Searching for the elusive targets of farnesyltransferase inhibitors. Nature Rev. Cancer 3, 945–951 (2003).
Zhu, K., Hamilton, A. D. & Sebti, S. M. Farnesyltransferase inhibitors as anticancer agents: current status. Curr. Opin. Invest. Drugs 4, 1428–1435 (2003).
End, D. W. et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 61, 131–137 (2001).
van Cutsem, E. et al. Phase III trial comparing gemcitabine + R115777 (Zarnestra) versus gemcitabine + placebo in advanced pancreatic cancer (PC). Proc. Am. Soc. Clin. Oncol. 21, A517 (2002).
Liu, A., Du, W., Liu, J. P., Jessell, T. M. & Prendergast, G. C. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol. Cell. Biol. 20, 6105–6113 (2000).
Katz, M. E. & McCormick, F. Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 7, 75–79 (1997).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002). Seminal discovery of somatic mutations of BRAF in human cancer. This report described the prevalence of BRAF mutations in melanoma and led to BRAF being viewed as a promising target for drug development for this patient population.
Satyamoorthy, K. et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 63, 756–759 (2003).
Rajagopalan, H. et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 418, 934 (2002).
Yuen, S. T. et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 62, 6451–6455 (2002).
Singer, G. et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl Cancer Inst. 95, 484–486 (2003).
Cohen, Y. et al. BRAF mutation in papillary thyroid carcinoma. J. Natl Cancer Inst. 95, 625–627 (2003).
Kimura, E. T. et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC–RAS–BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 63, 1454–1457 (2003).
Karasarides, M. et al. B-RAF is a therapeutic target in melanoma. Oncogene 23, 6292–6298 (2004). Reports the use of RNA interference to demonstrate that BRAF depletion impairs ERK signalling and proliferation in melanoma cells, unlike ARAF and RAF1 depletion. These findings strengthened the argument for developing a BRAF-targeted therapeutic agent against melanoma.
Tuveson, D. A., Weber, B. L. & Herlyn, M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell 4, 95–98 (2003).
Bollag, G., Freeman, S., Lyons, J. F. & Post, L. E. Raf pathway inhibitors in oncology. Curr. Opin. Invest. Drugs 4, 1436–1441 (2003).
Lee, J. T. & McCubrey, J. A. BAY-43-9006 Bayer/Onyx. Curr. Opin. Invest. Drugs. 4, 757–763 (2003).
Hotte, S. J. & Hirte, H. W. BAY 43-9006: early clinical data in patients with advanced solid malignancies. Curr. Pharm. Des. 8, 2249–2253 (2002).
DeGrendele, H. Activity of the Raf kinase inhibitor BAY 43-9006 in patients with advanced solid tumors. Clin. Colorectal Cancer 3, 16–18 (2003).
Verheul, H. M. & Pinedo, H. M. Vascular endothelial growth factor and its inhibitors. Drugs Today (Barc) 39 (Suppl. C), 81–93 (2003).
Hilger, R. A. et al. ERK1/2 phosphorylation: a biomarker analysis within a phase I study with the new Raf kinase inhibitor BAY 43-9006. Int. J. Clin. Pharmacol. Ther. 40, 567–568 (2002).
Chong, H., Vikis, H. G., Guan, K. L. Mechanisms of regulating the Raf kinase family. Cell Signal. 15, 463–469 (2003).
Mercer, K. E. & Pritchard, C. A. Raf proteins and cancer: B-Raf is identified as a mutational tartet. Biochim. Biophys. Acta 1653, 25–40 (2003).
Wan, P. T. C. et al. Mechanism of activation of the RAF–ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004). Describes the crystal structure of wild-type and oncogenic BRAF kinase domains. This important study provides insight into how BRAF is activated and how mutations in this protein lead to tumorigenesis.
Dibb, N. J., Dilworth, S. M. & Mol, C. D. Switching on kinases: oncogenic activation of BRAF and the PDGFR family. Nature Rev. Cancer 4, 718–727 (2004).
Cowley, S., Paterson, H., Kemp, P. & Marshall, C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH3T3 cells. Cell 77, 841–852 (1994).
Mansour, S. J. et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265, 966–970 (1994). Although MEK is not an oncogene, this study showed that constitutively activated MEK possesses transforming activity, providing the impetus for targeting the MAPK pathway in the development of molecular-targeted drugs.
Hoshino, R. et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 18, 813–822 (1999).
Haystead, T. A., Dent, P., Wu, J., Haystead, C. M. & Sturgill, T. W. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 306, 17–22 (1992).
Sebolt-Leopold, J. S. MEK inhibitors: a therapeutic approach to targeting the Ras–MAP kinase pathway in tumors. Curr. Pharmaceutical Design 10, 1907–1914 (2004).
Favata, M. F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 (1998).
Sebolt-Leopold, J. S. et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nature Med. 5, 81–816 (1999). The first report of a small-molecule MEK inhibitor having efficacy in tumour-bearing mice. Importantly, pharmacodynamic assays linked antitumour efficacy with suppression of the MAPK pathway.
Allen, L. F., Sebolt-Leopold, J. S. & Meyer, M. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK). Semin. Oncol. 30, 105–116 (2003).
Rinehart, J. et al. Multicenter phase 2 study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon and pancreatic cancer. J. Clin. Oncol. 13 Oct 2004 (doi:10.1200/JCO.2004.01.185)
Ohren, J. et al. Structures of human MAP Kinase Kinase 1 (MEK1) and MEK2 reveal a novel mode of non-competitive kinase inhibition. Nature Struct. Biol. (in the press). Structural evidence that both MEK1 and MEK2 possess a unique inhibitor-binding pocket adjacent to the ATP-binding site. This explains the high degree of selectivity observed with MEK inhibitors in clinical development.
Bishop, A. C. A hot spot for protein kinase inhibitor sensitivity. Chem. Biol. 11, 587–591 (2004).
Tamborini, E. et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology 127, 294–299 (2004).
Yeung, K. et al. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol. Cell. Biol. 20, 3079–3085 (2000).
Wang, Y. et al. The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol. Cell. Biol. 22, 916–926 (2002).
Matheny, S. A. et al. Ras regulates assembly of mitogenic signaling complexes through the effector protein IMP. Nature 427, 256–260 (2004).
Farooq, A. & Zhou, M. -M. Structure and regulation of MAPK phosphatases. Cell. Signalling 16, 769–779 (2004).
Shapiro, P. S. & Ahn, N. G. Feedback regulation of Raf-1 and mitogen-activated protein kinase (MAP) kinase kinases 1 and 2 by MAP kinase phosphatase-1 (MKP-1). J. Biol. Chem. 273, 1788–1793 (1998).
Guan, K. L. et al. Negative regulation of the serine/threonine kinase B-Raf by Akt. J. Biol. Chem. 275, 27354–27359 (2000).
Zhang, B. H. et al. Serum- and glucocorticoid-inducible kinase SGK phosphorylates and negatively regulates B-Raf. J. Biol. Chem. 276, 31620–31626 (2001).
Sivaraman et al. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Invest. 99, 1478–1483 (1997).
El-Ashry et al. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene 15, 423–435 (1997).
Coutts A. S. & Murphy, L. C. Elevated mitogen-activated protein kinase activity in estrogen-nonresponsive human breast cancer cells. Cancer Res. 58, 4071–4074 (1998).
Donovan, J. C., Milic, A. & Slingerland, J. M. Constitutive MEK/MAPK activation leads to p27Kip1 deregulation and antiestrogen resistance in human breast cancer cells. J. Biol. Chem. 276, 40888–40895 (2001).
Lee, S. H. et al. Colorectal tumors frequently express phosphorylated mitogen-activated protein kinase. APMIS 112, 233–238 (2004).
Wang, H. & Chakrabarty, S. Platelet-activating factor activates mitogen-activated protein kinases, inhibits proliferation, induces differentiation and suppresses the malignant phenotype of human colon carcinoma cells. Oncogene 22, 2186–2191 (2003).
Vial, E. & Marshall, C. J. Elevated ERK–MAP kinase activity protects the FOS family member FRA-1 against proteasomal degradation in colon carcinoma cells. J. Cell Sci. 116, 4957–4963 (2003).
Ishino, K. et al. Enhancement of anchorage-independent growth of human pancreatic carcinoma MIA PaCa-2 cells by signaling from protein kinase C to mitogen-activated protein kinase. Mol. Carcinog. 34, 180–186 (2002).
Tan, X. et al. Relationship between the expression of extracellular signal-regulated kinase 1/2 and the dissociation of pancreatic cancer cells: involvement of ERK1/2 in the dissociation status of cancer cells. Int. J. Oncol. 24, 815–820 (2004).
Manzano, R. G. et al. CL100 expression is down-regulated in advanced epithelial ovarian cancer and its re-expression decreases its malignant potential. Oncogene 21, 4435–4447 (2002).
Gioeli, D., Mandell, J. W., Petroni, G. R., Frierson, H. F. Jr & Weber, M. J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 59, 279–284 (1999).
Bakin, R. E., Gioeli, D., Sikes, R. A., Bissonette, E. A. & Weber, M. J. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 63, 1981–1989 (2003).
Zayzafoon, M., Abdulkadir, S. A. & McDonald, J. M. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J. Biol. Chem. 279, 3662–3670 (2004).
Eisenmann, K. M., VanBrocklin, M. W., Staffend, N. A., Kitchen, S. M. & Koo, H. M. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 63, 8330–8337 (2003).
Jorgensen, K., Holm, R., Maelandsmo, G. M. & Florenes, V. A. Expression of activated extracellular signal-regulated kinases 1/2 in malignant melanomas: relationship with clinical outcome. Clin. Cancer Res. 9, 5325–5331 (2003).
Calipel, A. et al. Mutation of B-Raf in human choroidal melanoma cells mediates cell proliferation and transformation through the MEK/ERK pathway. J. Biol. Chem. 278, 42409–42418 (2003).
Vicent, S. et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br. J. Cancer 90, 1047–1052 (2004).
Mawrin, C. et al. Prognostic relevance of MAPK expression in glioblastoma multiforme. Int. J. Oncol. 23, 641–648 (2003).
Meng, X. W. et al. Central role of Fas-associated death domain protein in apoptosis induction by the mitogen-activated protein kinase kinase inhibitor CI-1040 (PD184352) in acute lymphocytic leukemia cells in vitro. J. Biol. Chem. 278, 47326–47339 (2003).
Milella, M., Kornblau, S. M. & Andreeff, M. The mitogen-activated protein kinase signaling module as a therapeutic target in hematologic malignancies. Rev. Clin. Exp. Hematol. 7, 160–190 (2003).
Lunghi, P. et al. Downmodulation of ERK activity inhibits the proliferation and induces the apoptosis of primary acute myelogenous leukemia blasts. Leukemia 17, 1783–1793 (2003).
Carter, C. et al. Anti-tumour efficacy of the orally active Raf kinase inhibitor BAY 43-9006 in human tumour xenograft models. Proc. Annu. Meet. Am. Assoc. Cancer Res. 42, A4954 (2001).
Wallace, E. et al. Preclinical development of ARRY-142886, a potent and selective MEK inhibitor. Proc. Annu. Meet. Am. Assoc. Cancer Res. 45, A3891 (2004).
Acknowledgements
We gratefully acknowledge J. Ohren for providing the structural diagrams and for his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Both authors are employed by Pfizer Global Research and Development. Pfizer is widely known to have an active interest in the clinial development of MEK inhibitors — a subject that is included in this review.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
Glossary
- ISOPRENOID
-
A 15-carbon farnesyl lipid modification required for membrane localization and activity of RAS and other signalling proteins.
- IC50
-
The concentration of a drug required to inhibit target activity by 50%.
- NON-COMPETITIVE INHIBITOR
-
A kinase inhibitor that does not bind to or interfere with the ATP-binding site of an enzyme.
Rights and permissions
About this article
Cite this article
Sebolt-Leopold, J., Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4, 937–947 (2004). https://doi.org/10.1038/nrc1503
Issue Date:
DOI: https://doi.org/10.1038/nrc1503
This article is cited by
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy
Molecular Cancer (2023)
-
Vitamin C intake and colorectal cancer survival according to KRAS and BRAF mutation: a prospective study in two US cohorts
British Journal of Cancer (2023)
-
Mechanism of TNFα-induced downregulation of salt-inducible kinase 2 in adipocytes
Scientific Reports (2023)
-
Coadaptation fostered by the SLIT2-ROBO1 axis facilitates liver metastasis of pancreatic ductal adenocarcinoma
Nature Communications (2023)
-
The cardiac glycoside ZINC253504760 induces parthanatos-type cell death and G2/M arrest via downregulation of MEK1/2 phosphorylation in leukemia cells
Cell Biology and Toxicology (2023)