Abstract
Chronic stress leads to heightened affective behaviors, and can precipitate the emergence of depression and anxiety. These disorders are associated with increased amygdala activity. In animal models, chronic stress leads to increased amygdala-dependent behaviors, as well as hyperactivity of amygdala neurons. However, it is not known whether increased excitatory synaptic drive after chronic stress contributes to hyperactivity of basolateral amygdala (BLA; comprised of basal, lateral, and accessory basal nuclei) neurons. This study tested whether repeated stress causes an increase in excitatory drive of basal amygdala (BA) neurons in vivo, and whether this is correlated with an increase in the number of dendritic spines and a shift in dendritic distribution. Using in vivo intracellular recordings, this study found that repeated restraint stress caused an increase in the frequency of spontaneous excitatory synaptic events in vivo, which correlated with the number of dendritic spines in reconstructed neurons. Furthermore, parallel changes in the kinetics of the synaptic events and the distribution of spines indicated a more prominent functional contribution of synaptic inputs from across the dendritic tree. The shift in spine distribution across the dendritic tree was further confirmed with the examination of Golgi-stained tissue. This abnormal physiological drive of BA neurons after repeated stress may contribute to heightened affective responses after chronic stress. A reduction in the impact of excitatory drive in the BA may therefore be a potential treatment for the harmful effects of chronic stress in psychiatric disorders.
Similar content being viewed by others
INTRODUCTION
A number of psychiatric disorders, including depression and anxiety, can be precipitated or exacerbated by stressors (Heim and Nemeroff, 2001; Lupien et al, 2009). In the extreme, stress can induce post-traumatic stress disorder. These affective disorders have in common hyperactivity of the amygdala in male patients (Drevets et al, 1992; Breiter et al, 1996; Sheline et al, 2001; Thomas et al, 2001; Siegle et al, 2002; Davidson et al, 2003). The amygdala contributes to a range of emotion-related behaviors, and hyperactivity of the amygdala can contribute to disruptions in mood and emotion.
Repeated stress also leads to increased amygdala activity, observed as increased amygdala activation in humans exposed to chronic or traumatic stress (Shin et al, 1997; Armony et al, 2005; Protopopescu et al, 2005; Ganzel et al, 2007; van Wingen et al, 2011; Bogdan et al, 2012; Dannlowski et al, 2012), and an increase in immediate-early genes’ expression in neurons of the basolateral amygdala (BLA) area (comprised of basal, lateral, and basomedial nuclei) upon exposure to a novel stressor (Bhatnagar and Dallman, 1998; Mikics et al, 2008). Furthermore, neurons of the BLA are hyperactive and more responsive to afferents after stress (Adamec et al, 2005; Correll et al, 2005; Mozhui et al, 2010; Rosenkranz et al, 2010; Zhang and Rosenkranz, 2012). Hyperactivity of amygdala neurons after repeated stress can be caused by a number of alterations, including increased membrane excitability (Rosenkranz et al, 2010), which can lead to greater neuronal responsiveness, or by increased excitatory synaptic drive. Increased excitatory synaptic drive may occur via an increase of the number of excitatory inputs or a functional increase in the inputs registered. Synaptic spines are the sites of most excitatory inputs to BLA pyramidal neurons (Farb et al, 1992; Radley et al, 2007; Rademacher et al, 2010). The morphology of neurons in the basal nucleus (BA) of the BLA is sensitive to the effects of repeated stress. Repeated stress increases the number of spines and causes hypertrophy of dendrites of BA neurons (Vyas et al, 2002, 2006; Mitra et al, 2005; Hill et al, 2011, 2012; Adamec et al, 2012). However, it is not known whether repeated stress causes an increase of excitatory synaptic drive of BA neurons and a change in the characteristics of excitatory synaptic inputs. This study used Golgi staining to test if repeated restraint stress increases spine number and shifts their distribution, and in vivo intracellular recordings to test whether repeated stress leads to parallel increases of in vivo excitatory synaptic activity and a change in the properties of excitatory synaptic. Because of their sensitivity to repeated stress, BA neurons were the focus of this study.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of Rosalind Franklin University of Medicine and Science, and followed the Guide for the Care and Use of Laboratory Animals published by the US National Research Council.
Male Sprague–Dawley rats (Harlan; age 8–9 weeks at start) were group housed (2–3 per cage) in an animal facility with controlled climate, 12 : 12 hours light : dark schedule and food available ad libitum. To expose rats to repeated stress, they were placed in a restraint hemicylinder for 20 min per session, one session per day, for 7 out of 9 consecutive days (Figure 1a). This schedule of restraint exposure was previously shown to increase adrenal gland weight and reduce exploration in the elevated plus maze (Rosenkranz et al, 2010; Zhang and Rosenkranz, 2012); furthermore, it reduces intersession habituation to restraint, which would otherwise be prominent (Kant et al, 1985; Stamp and Herbert, 1999). A control group was handled in the same manner as the restraint group, except that they remained in a transparent cage with bedding, instead of a restraint cylinder. The total amount of handling between groups was equivalent. All further experiments were performed one day after the final restraint or control session. Confirming the effectiveness of the repeated restraint protocol in this study, the adrenal weight was greater in the stress group than in the control group (normalized to body weight: control, 0.099±0.003, n=40; stress, 0.113±0.002, n=41; p=0.0008, t=3.47, d.f.=79, two-tailed unpaired t-test).
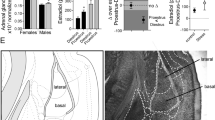
Repeated restraint protocol and basal amygdala nucleus diagram. (a) The repeated restraint protocol utilized 5 consecutive days of restraint or control handling (black rectangles) followed by 2 days of handling (white rectangles), and then 2 days of restraint or control handling (black rectangles). One day following the final restraint or handling session (white oval), rats were prepared for in vivo electrophysiology or Golgi staining. (b) The basal nucleus (basal amygdala) of the basolateral amygdala, defined by Paxinos and Watson (1998) in atlas coordinates (left) and Golgi-stained tissue (right).
Electrophysiology
In vivo intracellular electrophysiological recordings were obtained from the basal nucleus of the amygdala. Rats were anesthetized with an initial dose of 8% chloral hydrate (all chemicals were from Sigma-Aldrich, St Louis, MO, unless noted otherwise), and supplemented as necessary. Burr holes were drilled over the right and left amygdale (centered at 3.0 caudal, 5.0 lateral to bregma). A stainless-steel screw was imbedded in one burr hole for EEG recordings, and the intracellular recording electrode was lowered through the other. Electrodes were filled with 1–2% neurobiotin in 2 M potassium acetate (40–60 M( ) resistance measured in vivo). In a subset of experiments, the chloride channel blocker 4,4′-dinitrostilbene-2,2′-disulfonate (DNDS, 0.5 mM; Invitrogen, Grand Island, NY) or DNDS+CsCl (0.5 mM) was included in the recording electrode.
Series resistance was compensated using built-in amplifier bridge circuitry (IR-183; Cygnus Technology, Delaware Water Gap, PA). Neurons were excluded if their action potentials did not overshoot 0 mV, if they displayed firing characteristics inconsistent with BLA pyramidal neurons in vivo (Lang and Pare, 1997; Rosenkranz and Grace, 2002), or if their resting membrane potential was more depolarized than −60 mV. Periods of membrane potential were collected and spontaneous synaptic events were detected using a sliding template (Axograph X, Sydney Australia; as described in Rosenkranz, 2011, 2012). The detection threshold was set at three times larger than the standard deviation (SD) of the noise, that is, threshold=template scaling factor/√(SSE/(N−1). Synaptic events were visually examined to verify their inclusion for analysis. The rise time was only measured from synaptic events with intervals of at least 5 ms, and the decay half-width was only measured from synaptic events with at least 25-ms interval before subsequent events. To verify the ability to discriminate these events from noise, the noise was measured during quiescent periods of recordings (periods with no observable synaptic activity). To quantify the noise, the SD of the membrane potential during quiescent activity was quantified. A minimum of 10 segments of quiescent activity, each >150-ms duration, were included for analysis from each neuron. The SD was plotted against the amplitude histogram of detected events from control and stress groups to verify separation of noise from synaptic events. Spontaneously occurring clusters of synaptic events were also quantified. To be characterized as a cluster of postsynaptic potentials (PSPs), the event had to fulfill several criteria: the event had to last longer than 200 ms before returning to within 90% of the baseline membrane potential, the event had to contain clear synaptic events, and the peak amplitude had to remain at least × 3 the average amplitude of individual PSPs at that membrane potential. To be considered a second cluster of PSPs, the initial cluster had to return to baseline membrane potential, and there had to be a separation of at least 200 ms between clusters. A minimum of 60 clusters and 300 PSPs were measured from each neuron. Using these criteria, peak amplitude of each cluster of PSPs was quantified as the average peak amplitude of the clusters. At the conclusion of experiments, brains and adrenal glands were removed. Adrenal glands were weighed (wet). Brains were histologically processed for neurobiotin to confirm the recording site and allow reconstruction of the recorded neuron. Brains were soaked in 4% paraformaldehyde for at least 24 h, followed by sucrose. Brains were sliced on a freezing microtome at 60 μm. Staining for neurobiotin was performed using a standard ABC peroxidase assay (Vector Laboratories), followed by staining with a mix of cresyl violet and neutral red. Neurons were excluded from analysis if they were found to lie outside the BA, or if their morphology was inconsistent with pyramidal neurons (McDonald, 1982).
Golgi Stain
In a separate group of rats, Golgi-Cox staining of brain tissue was performed using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Columbia, MD), following the manufacturer’s suggested protocol. Rodents were deeply anesthetized with chloral hydrate, as above, decapitated, and the brain was rapidly removed. The brain was blocked and placed immediately into impregnation solution. The impregnation solution was changed after 24 h and then stored in the dark for 15–18 days at room temperature. Brain tissue was then transferred to solution C of the FD Rapid GolgiStain Kit for 24 h at 4 °C. After replacing the solution with fresh solution C, the tissue was stored at 4 °C for 7 days. Brains were sectioned (100 μm thickness, Leica SM 2000R microtome), and slices were collected in 20% sucrose in 0.1 M phosphate buffer at room temperature. Slices were mounted on gelatinized slides and air dried (25 min–1 h), and then rinsed in ddH2O (two times, 4 min each rinse). Slides were dehydrated in 50, 75, and 95% ethanol for 4 min each, and then in 100% ethanol four times for 3 min each. Slides were cleared with xylene (three times, 4 min each), and then coverslipped with Permount. Slides were allowed to dry overnight. Throughout the staining and subsequent study, slides were protected from light.
Neuronal Reconstruction
Neurobiotin-filled or Golgi-stained neurons were reconstructed using the Neurolucida software (MBF Bioscience, Williston, VT) under bright-field illumination using the × 100 objective of a Nikon Eclipse E400 microscope. Only neurons that appeared to be completely filled were utilized. Thus, secondary and tertiary dendrites had to be visible, and spines had to be visible. In addition, there could be no breaks in the dendrites, except breaks when a dendrite can be followed into an adjacent section. Neurons were selected based on morphology consistent with BLA principal neurons (obvious primary dendrites and spines), localization within the BA (Figure 1b). Reconstructions were performed by an individual blind to treatment conditions. Aspiny neurons that displayed small somata with few dendrites or large somata with bipolar primary dendrites were not included in this analysis. The dendritic length and total number of spines in each dendritic segment were quantified for comparison. In addition, the relative distribution of spines across the dendritic tree was quantified as ((spine numberBr)÷(spine numberTot)), where spine numberBr is the the number of spines at a specific branch order, and spine numberTot is the total number of spines on that neuron. Branch order was measured centrifugally, so that at each branch point both the parent and dendritic branch increase branch order. Photographs were acquired at × 10, × 20, or × 100 magnification from control and stress groups under similar light conditions. In the images displayed in figures, only the size and contrast of the images had been adjusted.
Statistical Analysis
Neurons were excluded from morphological analysis if they did not display morphological aspects of BA pyramidal-like neurons (as described above), or if their mean length or spine density was >2 SD from the mean. Similarly, neurons were excluded from analysis if measures of spontaneous synaptic activity (amplitude, frequency, rise time, half-width) were >2 SD from the mean. This led to the exclusion of five neurobiotin-filled neurons (two from the control group and three from the stress group). A total of 56 neurons were analyzed after Golgi staining (from 30 rats), and 66 neurons from 51 rats for in vivo intracellular recordings. Comparisons of multiple factors were examined using a two-way ANOVA. An α-level of 0.05 was considered significant. For planned comparisons of single parameters between two groups, two-tailed unpaired t-tests were used. Data were tested for normality of distribution (Kolmogorov and Smirnov (K–S) test) and for equality of the SD (Bartlett’s test). If data failed these tests, non-parametric tests were planned. Statistical tests were performed using Igor Pro (Wavemetrics, Lake Oswego, OR) or Prism 6 software (GraphPad Software, La Jolla, CA). All values are expressed as the mean±SEM.
RESULTS
Repeated Stress Shifts Spine Distribution in Golgi-Stained BA
Previous studies have demonstrated that repeated stress increases BA neuronal dendritic length and number of spines (Vyas et al, 2002, 2006; Mitra et al, 2005; Hill et al, 2011, 2012). However, it is not clear whether there is a shift in the distribution of spines across the dendrites. To examine the distribution of spines, Golgi-Cox staining was performed. BA principal neurons displayed prominent primary dendrites, multiple branch orders, and were spiny, consistent with previous descriptions (eg McDonald, 1982; McDonald et al, 1982; Figure 2a). Repeated restraint stress caused an increase of dendritic length (Figure 2b; Sholl analysis: p=0.0016, two-way repeated measures ANOVA, F(1,54)=11.0, n=15 rats per group, 28 neurons per group; total dendritic length: control, 1447±95 μm; stress, 2082±166 μm; p=0.0016, two-tailed t-test, t=3.32) and number of spines (Figure 2; Sholl analysis: p=0.0021, two-way repeated measures ANOVA, F(1,54)=10.5, n=12 rats per group, 28 neurons per group; total spines: control, 328.8±33.3; stress, 542.3±52.9; p=0.0012, two-tailed t-test, t=3.42) of BA neurons in Golgi-stained tissue. Preliminary examination of the Sholl plots indicate increased spine number at intermediate and distal sites (>100 μm from soma), but not close to the soma (<60 μm from the soma), warranting further examination. The distribution of spines was quantified as the proportion of spines at branches of increasing order from the soma. Branch order was used instead of distance to control for the increased dendritic length after repeated stress. There was a shift in the distribution of spines such that a higher proportion of spines were located at more distal branches after repeated restraint stress compared with control handled rats (Figure 2e; Gaussian best fit to different curves, p<0.0001, F=11.6; control mean, 3.0±0.08, mean SD=1.38; stress mean, 3.5±0.13, mean SD=1.93).
Repeated stress increases basal amygdala pyramidal neuron dendritic length and spine number: Golgi stain. Basal amygdala spiny principal neurons we reconstructed after Golgi-Cox staining. (a) After repeated restraint stress, principal neurons of the basal amygdala tended to have more elaborate dendritic trees and higher number of spines. (b) Sholl analysis demonstrated that repeated restraint led to a significantly greater dendritic length of basal amygdala principal neurons, particularly at intermediate and more distal distances, and a greater average total dendritic length across the population. (c) Sholl analysis demonstrated that repeated restraint caused a greater number of spines at intermediate and more distal distances, and greater average number of spines/neuron across the population. (d) Repeated restraint stress did not lead to a significantly greater spine density. (e) Repeated restraint stress caused a shift in the distribution of spines across the dendritic branches, with a greater proportion of spines at more distal locations (higher branch orders). *Significant main effect of stress in a two-way repeated-measures analysis of variance.
Principal neurons from the BA that were recorded in vivo and filled with neurobiotin displayed similar morphological changes after repeated stress. Reconstructed neurobiotin-filled neurons included in this analysis (22 reconstructed neurons per 20 rats) displayed features typical of BA pyramidal neurons, including large soma size, thick primary dendrites (Figure 3a), and spines (Figure 3b). Repeated stress caused an increase in total dendritic length (Figure 2c; control, 1881±238 μm, n=10; stress, 3064±358 μm; n=12, p=0.016, t=2.63, two-tailed unpaired t-test) and total number of spines (Figure 3d; control, 269.1±36.9 spines, n=10; stress, 442.3±45.7, n=12; p=0.0095, t=2.87, two-tailed unpaired t-test) compared with controls. Sholl analysis indicated that the increase in dendritic length (p=0.0098, two-way repeated-measures ANOVA, F(1,20)=8.15) and the increase in spine number (p=0.0096, two-way repeated-measures ANOVA, F(1,20)=8.20) occurs over a range of intermediate and distal distances from the soma, but not close to the soma (<60 μm from soma). Because spines are the site of most glutamatergic inputs to neurons of the BA (Farb et al, 1992; Radley et al, 2007; Rademacher et al, 2010), the increased number of spines is consistent with increases in the number of glutamatergic synapses onto BA neurons. There was no significant increase in the spine density in the repeated stress group compared with controls (Figure 3e; control, 1.42±0.11 spines per 10 μm, n=10; stress, 1.76±0.34 spines per 10 μm, n=12; p=0.40, t=0.87, two-tailed unpaired t-test).
Repeated stress increases basal amygdala pyramidal neuron dendritic length and spine number: In vivo neurobiotin filling. Basal amygdala principal neurons were filled with neurobiotin and reconstructed. (a) Repeated restraint stress increased the dendrites of basal amygdala neurons, observed here in neurons from the control (left) and stress group (right) Magnification in both images is × 20 objective. (b) Repeated restraint stress increased the number of spines, observed here on dendrites of a neuron from the control group (left) and stress group (right) at approximately the same distance from the soma (∼100 μm). Magnification in both images is × 100. (c) Repeated restraint stress caused an increase in the total dendritic length (left) and this increase is most apparent at intermediate and more distal distances from the soma in a Sholl analysis. (d) Repeated restraint stress caused an increase in the total number of spines/neuron, particularly apparent at intermediate and more distal distances from the soma. (e) Repeated restraint stress did not cause an increase of the spine density. In all panels, black represents the control group and grey represents the stress group. *indicates p<0.05 in a two-tailed unpaired t-test. †indicates significant main effect of stress in a two-way repeated measures ANOVA.
Repeated Stress Increases Excitatory Synaptic Input
To test whether repeated stress causes an increase of excitatory inputs, and whether there is a functional significance to the increased spines and the change in spine distribution, the synaptic activity of BA neurons was recorded in vivo. In chloral hydrate anesthetized rats, BLA neurons in vivo display periodic spontaneous synaptic events, (Figure 4a; Rosenkranz, 2011, 2012). These synaptic events tend to occur in clusters (at approximately 1 Hz) of PSPs that summate together (Figure 3b). Initial examination indicated that clusters of spontaneous synaptic events had a significantly larger amplitude after repeated stress compared with controls (Figure 4a and c; peak amplitude measured near −70 mV; control, 11.3±0.6 mV, n=14; stress, 14.9±0.9 mV, n=17; p=0.002, t=3.36, two-tailed unpaired t-test). To derive a measure of the membrane fluctuations caused by synaptic events that does not depend on the criteria for definition of clusters, the SD of the mean membrane potential was measured. This measure also indicated a greater degree of spontaneous synaptic activity after repeated stress (Figure 4c; measured near −70 mV; control, 2.36±0.15, n=14; stress, 3.09±0.21, n=17; p=0.011, t=2.71, two-tailed unpaired t-test). Consistent with this, examination of the membrane potential distribution among the neurons sampled indicated a wider range of membrane potentials after repeated stress (Figure 4d; measured near −70 mV).
Repeated stress increases in vivo spontaneous synaptic input. (a) Basal amygdala principal neurons display spontaneous fluctuations of the membrane potential in anesthetized rats, as observed in recordings of membrane potential over time. Repeated restraint stress causes a higher degree of fluctuation of the membrane potential. (b) These fluctuations are composed of spontaneously occurring synaptic events that tend to occur in groups (or clusters), observed here in overlays of three clusters. In these overlays, repeated stress increased the amplitude of these clusters. (c) Average peak amplitude of the clusters is significantly greater after repeated stress. To further confirm this, the average standard deviation (SD) of the mean membrane potential was determined for each group. Repeated stress caused an increase in the average amplitude of the SD. (d) The wider SD can be observed in a non-biased manner by examining the histograms of the deviation of the membrane potential from the resting membrane potential (0 in this plot). Data used for this histogram include the membrane potential from 60-s epochs of all neurons. In all panels, black represents the control group and gray represents the stress group. *p<0.05 in a two-way unpaired t-test.
To determine what factors underlie the increased amplitude of clusters, they were examined in more detail. These clusters of synaptic activity are comprised of synaptic events that can be measured (Figure 5a and b). Because there are a large number of events that occur in a short time window, presumably at different distances from the soma, we cannot assume that each measured event corresponds to one excitatory PSP (EPSP) from one synapse. The measured events are likely composed of single and multiple synaptic events from synapses at varying distances from the soma, leading to a wide range of measured amplitudes. Therefore, these studies do not assume that the PSPs are single synaptic events. However, this analysis assumes that the detectability of events remains similar across experiments. To verify this assumption, the noise level compared with the amplitude of these detected events was compared between control and stress groups (Figure 5c). The noise of recordings was measured as the SD of the membrane potential during quiescent periods with no observed synaptic activity. There was no significant difference in the quiescent SD between control and stress groups (Figure 5c; SD: control, 0.18±0.01 mV, n=14; stress, 0.18±0.01 mV, n=17; p=0.96, t=0.05, two-tailed unpaired t-test). Furthermore, there was clear separation between the amplitude of noise and the amplitude of detected synaptic events plotted as a frequency histogram (Figure 5c, left). For simplicity, and to distinguish them from clusters of events, these will be referred to as individual synaptic events (despite the likely multievent nature of many events). There was not a significant difference in the amplitude of the synaptic events that comprise these clusters, between control and stress groups (Figure 5b; control, 3.06±0.16 mV, n=14; stress, 3.32±0.22 mV, n=17; p=0.36, t=0.92, two-tailed unpaired t-test). If increased amplitude of individual synaptic events is not observed, the increase in the amplitude of clusters could be caused by increased summation of individual synaptic events. There are two potential sources for this increased summation that we can measure: (1) increased duration of individual synaptic events, allowing greater window for summation, or (2) increased number of synaptic potentials that summate during the event cluster. We found evidence for the latter. Repeated stress caused an increase in the frequency of individual synaptic events as a whole (Figure 5d; control, 19.2±1.8 Hz, n=14; stress, 27.6±1.7 Hz, n=17; p=0.0019, t=3.42, two-tailed unpaired t-test), and in the number of synaptic events within each cluster of synaptic activity (Figure 5e; control, 16.8±0.7 events per cluster, n=14; stress, 22.2±1.2 events per /cluster, n=17; p=0.0012, t=3.59, two-tailed unpaired t-test). There was a trend towards increased PSP duration after repeated stress, although it did not reach statistical significance (Figure 5f; half-width: control, 10.45±0.92 ms, n=14; stress, 12.41±0.79 ms, n=17; p=0.11, t=1.64, two-tailed unpaired t-test).
Repeated stress increases the frequency of individual synaptic events. (a) The individual synaptic events that compose the clusters can be isolated. There was no significant effect of repeated stress on the amplitude of these individual synaptic events, as seen here from an overlay of all the synaptic events that occurred in a 60-s time window from representative examples. (b) When averaged, there was no significant effect of repeated stress on the amplitude of individual synaptic events. (c) Overlays of four consecutive ‘quiescent periods’ during recordings (left) demonstrate low levels of background noise, quantified as standard deviation (SD) of the quiescent membrane potential (middle), which displayed separation from synaptic events plotted as a frequency histogram of event amplitude (right; vertical gray bar indicates±SD amplitude of the quiescent period) in two representative examples. (d) Repeated stress caused an increase in the frequency of individual synaptic events, as shown by a rightward shift in the cumulative probability of the instantaneous frequency of all synaptic events from all neurons, and by an increase in the average frequency of synaptic events. (e) Repeated stress caused an increase in the number of synaptic events per cluster, averaged across groups. (f) There was no significant effect of repeated stress on the half-width of synaptic events. In all panels, black represents the control group and gray represents the stress group. *p<0.05 in a two-way unpaired t-test.
Frequency of Isolated Excitatory Synaptic Events
At the resting membrane potential under these recording conditions, both fast EPSPs and inhibitory PSPs are in the depolarizing direction, making it difficult to verify that the events recorded are in fact reflective of excitatory inputs. To determine whether the effects of repeated stress on the frequency of spontaneous PSPs was a reflection of excitatory events, spontaneous synaptic events were measured near the chloride reversal potential, to minimize the amplitude of fast GABAergic events (Vm −55 mV; Figure 6a). We found that, even under these conditions, there was a significantly greater frequency of synaptic events following repeated stress compared with controls (Figure 6b; control, 15.6±1.5 Hz, n=9; stress, 22.8±2.6 Hz, n=11; p=0.032, t=2.32, two-tailed unpaired t-test). To further confirm this using another approach, in a separate group of neurons, the chloride channel blocker DNDS was included in the intracellular pipette to block fast GABAergic events (Figure 6a and c). In these conditions, the extrapolated reversal potential of these events is close to the expected reversal potential for glutamatergic inputs (Rosenkranz, 2012). Here too, there was a significantly greater frequency of spontaneous events after repeated stress compared with control rats (Figure 6c; control, 19.2±2.3 Hz, n=7; stress, 26.7±2.3 Hz, n=9; p=0.041, t=2.25, two-tailed unpaired t-test).
Repeated stress increases the frequency of excitatory events. The clusters of synaptic events could be composed of both excitatory and inhibitory synaptic events. (a) To isolate excitatory postsynaptic potentials, two approaches were utilized. Neurons were held near −55 mV, to minimize the amplitude of inhibitory events (left) or neurons were recorded with 4,4′-dinitrostilbene-2,2′-disulfonate (DNDS) in the recording pipette to block fast inhibitory events (right; indicating the effectiveness of DNDS at blocking fast GABAergic inhibitory postsynaptic potential). Note the occasional downward deflections of the membrane potential at −55 mV under control conditions (left), and their absence in the presence of DNDS (right). Action potentials are truncated for clarity. (b) When neurons were held near −55 mV, a significant effect of repeated stress was still observed on the frequency of synaptic events. (c) Similarly, when γ-aminobutyric acid-A channels were blocked with intracellular DNDS, repeated stress significantly increased the frequency of synaptic events. (d) When cesium (Cs+) was included in the pipettes with DNDS, there was a significant effect of repeated stress on the frequency of synaptic events (left). There was no significant effect of repeated stress on the amplitude of individual excitatory postsynaptic potential with Cs+ and DNDS in the pipette (right). In all panels, black represents the control group and gray represents the stress group. *p<0.05 in a two-way unpaired t-test.
One difficulty in interpretation arises because of the expected smaller amplitude of more distal events, coupled with dendritic filtering of these events. To reduce these concerns, cesium (Cs+) was included in the pipette, making dendrites electrotonically more compact, along with DNDS. In the presence of Cs+, we found an increase in the amplitude of clusters (peak amplitude: control, 11.3±0.7 mV, n=10; Cs+, 14.9±1.4 mV, n=9; p=0.035, t=2.30, two-tailed unpaired t-test), verifying the effectiveness of internal Cs+. With Cs+ present, there was still a significantly greater frequency of EPSPs after repeated stress compared with controls (Figure 6d; control, 21.7±2.6 mV, n=9; stress, 29.7±2.2 mV, n=10; p=0.032, t=2.31, two-tailed unpaired t-test). These data support an overall increase of excitatory synaptic input to BA pyramidal neurons after repeated stress.
Association Between Dendritic Spines and Synaptic Events
If the increase in frequency of EPSPs is caused by an increase in synaptic inputs formed onto new spines, one might expect to find a correlation between the frequency of events and the number of synaptic spines. We examined the correlation between the increased frequency of events and the increased number of spines in each neurobiotin-filled, reconstructed neuron. We found a correlation between the number of spines/neuron and the frequency of synaptic events (Figure 7a; overall r2=0.55, slope=12.9, significantly different than 0 at p<0.001; control neurons r2=0.44, slope=15.2, significantly different than 0 at p=0.037; stress neurons r2=0.40, significantly different than 0 at p=0.029).
Repeated stress leads to a morphological and functional shift in the distribution of synaptic inputs. (a) The frequency of synaptic events was correlated with the number of synaptic spines. (b) The normalized number of spines per neuron varied by distance, and is shifted towards more distal locations (higher branch orders) after repeated stress. (c) Repeated stress changed the mean rise time of individual synaptic events, towards events with slower rise times, consistent with more distal synapses (left). There was a shift in the population distribution of average rise time values towards slower rise times after repeated restraint stress (right). (d) A cumulative histogram of the rise times of all synaptic events demonstrates that repeated stress causes a shift in the distribution of rise times towards slower events. In all panels, black represents the control group and gray represents the stress group. *p<0.05 in a two-way unpaired t-test.
Similar to Golgi-stained BA pyramidal neurons, there was a change in the distribution of inputs along the dendritic tree towards more distal locations after repeated stress (Figure 7b; spine number × dendritic branch order, Gaussian best fit to different curves, p<0.0001, F=13.8; mean control, 2.48±0.07 branch order, SD=1.05; mean stress, 3.18±0.08 branch order, SD=1.37). Synaptic inputs at different locations along the proximal–distal range of dendrites often have differing kinetics (Magee, 2000). Specifically, inputs that are relatively more distal tend to have a slower rise time (Turner, 1988; Andreasen and Lambert, 1998; Rosenkranz and Johnston, 2007). If there is a change in the distribution of spines along the dendritic tree, it is expected that there would be a corresponding change in the distribution of the rise times of synaptic events that may occur at these spines. There was a significant change in the values of the rise times of synaptic events, observed as a significantly longer mean rise time (Figure 7c; rise time: control, 4.2±0.3 ms, n=14; stress, 6.1±0.5 ms, n=17; p=0.0015, t=3.51, two-tailed unpaired t-test), a significant shift in the distribution of the mean rise time (Figure 7c; Gaussian best fit to different curves, p=0.041, F=3.88), and a shift in the distribution of rise time values of all synaptic events (Figure 7d; D=0.667, p=0.002, K–S test).
The shift in the distribution of spines and the rise times of synaptic events is consistent with a structural and functional shift towards a contribution of more distal synaptic events. To determine whether the increased frequency of synaptic events is heavily attributable to the proportional increase in more distal synapses, we correlated the frequency of events with the number of spines at a range of distances from the soma for each neuron (Figure 8a). When analyzed in this manner, there was a significant positive correlation between the frequency of synaptic events and the number of spines in more proximal dendrites in the control group (Figure 8a; slope significantly non-zero, p<0.01 at 10–50 μm, 60–100 μm, others NS), but in the stress group there was a stronger positive correlation between frequency of synaptic events and the number of spines in more distal dendrites (Figure 8a; slope significantly non-zero, p<0.01 at 110–150 μm, 160–200 μm, 210 μm and above, but not at 10–50 μm; see Figure 8b for summary). Examination of the correlation as a function of distance (comparison of curve fits) demonstrated that the frequency of events is more readily attributed to proximal spines in the control group (0–100 μm) and more distal spines in the stress group (100–200 μm; nonlinear goodness-of-fit, best fit by second-order polynomials with significantly different parameters, Y=b0+b1 × X+b2 × X2, F(3,4)=8.62, p<0.001). This is consistent with a greater impact of more distal synaptic inputs after repeated stress.
Repeated stress causes a shift in the correlation of spines with the frequency of synaptic events. The number of spines was correlated with the number of synaptic events. If a greater proportion of spines at a specific distance contribute more to the measured synaptic activity, a higher correlation between spine numbers at postsynaptic potential frequency is expected at that specific distance. (a) In the control group, there was a significant correlation between the frequency of synaptic events and number of synapses, but only with synapses at more proximal locations (10–100 μm). After repeated stress, there was a significant correlation between the frequency of synaptic events and the number of spines at relatively more distal locations (110 μm and above). Correlation r2 values >0.45 are highlighted with gray box. (b) When the correlation coefficients are plotted by distance from the soma, an opposite pattern emerges between control and stress groups, with high correlation coefficients at proximal locations in the control group and high correlation coefficients at intermediate and more distal locations in the stress group. This indicates that the number of synaptic events was more attributable to intermediate and relatively distal synapses after repeated stress. As above, correlation r2 values >0.45 are highlighted with gray box.
DISCUSSION
Repeated stress leads to abnormal production of affective behaviors that are modulated by the BLA (Conrad et al, 1999; Vyas and Chattarji, 2004; Wood et al, 2008; Atchley et al, 2012). Furthermore, repeated stress leads to an increase of dendritic length and spine number in BLA neurons (Mitra et al, 2005; Vyas et al, 2006; Mozhui et al, 2010; Hill et al, 2011). Repeated stress also leads to an increased responsiveness of BLA neurons (Adamec et al, 2005; Correll et al, 2005; Mozhui et al, 2010; Rosenkranz et al, 2010), and an increase in MK801 binding in the BLA after chronic variable stress (Lei and Tejani-Butt, 2010), indicative of an increased number of NMDA-type glutamate receptors. A change in the number of spines might not necessarily lead to an increase of BA neuronal function if those spines are inactive or functionally weak. Our data demonstrate that, after repeated stress, there was a greater functional synaptic input associated with increased dendritic spines.
After repeated stress, there was a greater number of spines on BA neurons. Although there was an increase in spines across most of the neuron, there was a shift in the distribution of spines, such that a greater proportion of spines were at intermediate and more distal locations after repeated stress. Accompanying this change was an increase in the frequency of in vivo spontaneous EPSPs. Synaptic spines are the primary target of excitatory synaptic inputs, and there was a strong positive correlation between the number of spines and the frequency of spontaneous synaptic events. Therefore, this increased frequency may reflect an increased number of synapses. In addition, there was a shift in the distribution of the rise times of synaptic events, towards slower rise times. Because synaptic events that originate at more distal locations and propagate to the soma tend to have slower rise times (eg, Magee, 2000; Magee and Cook, 2000), this shift of rise times in our data is consistent with a greater proportion of synapses being located at more distal locations. However, several caveats that limit the interpretation of these results should be noted: (1) there is a wide distribution of spines (and presumably synapses) across the dendritic tree, with expected differences in kinetics and amplitude, perhaps with the most distal synapses being undetectable even in the presence of Cs+; (2) repeated stress may cause a change in membrane properties in some BA neurons (eg, Rosenkranz et al, 2010) that could influence the propagation of synaptic events to the soma; (3) the synaptic events that were measured cannot be proven to be unitary synaptic events, but may be comprised of multiple events. However, this shortcoming does not invalidate the correlations between this unit of synaptic activity and spines, but the non-unitary nature of these events will influence the accuracy of our results; and (4) while the majority of dendritic spines are occupied by asymmetric, glutamatergic inputs, a proportion of spines receive GABAergic symmetric synapses (McDonald et al, 2002; Muller et al, 2006, 2007). A change of GABAergic synaptic input would likely influence excitatory drive. While experiments in this study were able to measure excitatory drive, this study did not examine the effects of stress on inhibitory regulation. Furthermore, this study did not examine the duration of these effects of repeated restraint stress. A previous study found that dendritic hypertrophy of BLA neurons was not reversed after 21 days (Vyas et al, 2004), while the increased responsiveness of the BLA to stimuli after combat stress did return to baseline levels (van Wingen et al, 2012). Perhaps given enough recovery time, hypertrophy of BA neurons would revert back to control levels, or perhaps, the impact of stress and trauma depend on its severity, and could last longer in other human populations. However, even in the presence of these caveats, repeated restraint stress caused an increase in excitatory synaptic events that originated at more intermediate and distal distances from the soma.
Repeated stress increased the amplitude of periodic clusters of synaptic events without significant effects on the amplitude of small synaptic events. Instead, the frequency of these small synaptic events was increased after repeated stress. These periodic clusters of spontaneous events are likely synaptic in origin because their frequency is not voltage-dependent (Rosenkranz and Grace, 2002; Crane et al, 2009; Rosenkranz, 2012), nor are they abolished by blockade of a range of K+ or Ca2+ channels (Rosenkranz, 2011, 2012), nor is their duration reduced by voltage clamp near resting membrane potentials (Crane et al, 2009) as would be expected if they were due to intrinsic voltage-dependent oscillations. When blockers of GABA receptors are present, the reversal potential of these clusters is close to the expected reversal potential of glutamatergic synaptic events (Rosenkranz, 2012). Moreover, BLA neuronal firing, which occurs during some of these depolarizations, is time locked to the EEG of cortical regions that send excitatory afferents to the BLA (Pare et al, 1995a), and they can be mimicked by stimulation of certain excitatory afferents (Windels et al, 2010). Furthermore, in other regions that display similar clusters of synaptic activity, the clusters are time locked to the EEG and to other neurons (Contreras and Steriade, 1995), the clusters can be blocked with tetrodotoxin (Destexhe and Pare, 1999), which blocks axonal conduction and action potential-dependent release of neurotransmitter, clusters are comprised of events that are synaptic in origin (Haider et al, 2006), and when modeled in vitro they can be blocked with bath application of AMPA receptor antagonists (Sanchez-Vives and McCormick, 2000). Similar to BLA neuronal firing (Pare et al, 1995b; Gaudreau and Pare, 1996), the neuronal subthreshold membrane oscillatory repetition closely follows the cortical EEG (Rosenkranz, 2012), and indicates that this oscillatory behavior of these synaptic inputs may be due to oscillatory network activity in cortical regions that project to the amygdala during the anesthetized state. This is consistent with the state of other regions during anesthesia, sleep and quiet wakefulness (Steriade et al, 1993a; Lampl et al, 1999; Petersen et al, 2003; Volgushev et al, 2006; Poulet and Petersen, 2008).
BLA neurons display rapid responses to acute stressors. For instance, single footshocks acutely increase the firing of neurons in the LAT (Machne and Segundo, 1956; Romanski et al, 1993; Correll et al, 2005), but often acutely suppress the firing of neurons in the BA (Muramoto et al, 1993; Correll et al, 2005; Buffalari and Grace, 2007; Chen and Sara, 2007). Single stressors can also exert more persistent actions over minutes to hours. For instance, a series of footshocks over several minutes can lead to enhancement of auditory-evoked field potentials in the BLA (Garcia et al, 1998), and increased firing of BLA neurons (Pelletier et al, 2005). Longer stressors can also exert immediate actions (measurable immediately and within hours after the prolonged stress). Thus, longer periods of footshock (eg, 30 min) lead to a suppression of firing on BA neurons (Shors, 1999). However, 30 min of elevated platform stress, 45 min of forced swim, and 60 min of predator stress all result in an increase of excitatory drive of the BA (Vouimba et al, 2004, 2006; Kavushansky and Richter-Levin, 2006; Kavushansky et al, 2006). There is less information with regard to longer lasting effects of single stressors on BLA neuronal physiology. However, several studies indicate that a single stress session can increase excitatory drive measured one day later (Garcia et al, 1998; Vouimba et al, 2004; Adamec et al, 2005; Rodriguez Manzanares et al, 2005). Previous studies have found that a single stress does not cause a increase of spines in BLA neurons unless it is followed by an incubation period (Mitra et al, 2005). Therefore, the increased excitatory drive noted in the electrophysiology experiments after a single stress is not likely related to an increase of spines. Instead, it may be related to a decrease of inhibition (Isoardi et al, 2007), or an increase of release probability (Karst et al, 2010).
Our findings support increased synaptic drive after repeated stress, albeit with shifted proximal–distal dendritic distribution. A shift towards more distal synapses does not necessarily imply weaker synaptic drive. Overall, there were more synapses at both intermediate and distal locations after repeated stress. The highest correlations between the frequency of synaptic events and the number of spines were also shifted towards more distal locations after repeated stress. This supports the hypothesis that a greater amount of synaptic drive from intermediate and distal locations is able to propagate to the soma after stress. In other words, the soma is listening to a wider range of synapses after stress. A potential ramification of the longer kinetics of distal EPSPs, combined with their increased frequency, is that in the awake, asynchronous state, this flood of EPSPs may provide a greater tonic excitatory input to BA neurons. It is expected that a change in proximal inputs would exert a greater influence on neuronal firing than a change in distal inputs. The change in distal inputs itself may not strongly influence ongoing neuronal firing. However, changes in distal inputs exert significant effects on synaptic integration and synaptic plasticity (Schaefer et al, 2003; Larkum et al, 2004; Oviedo and Reyes, 2005; Williams, 2005), which will also ultimately lead to changes of neuronal firing. Furthermore, the dendritic structure itself contributes to computational ability of neurons (Mainen and Sejnowski, 1996; Jaffe and Carnevale, 1999; Vetter et al, 2001), and the presence and density of spines modifies propagation of signals (Shepherd et al, 1985; Segev and Rall, 1988; Baer and Rinzel, 1991; Jaslove, 1992). In other brain regions, such as the cortex and hippocampus, active properties of dendrites may reduce the differences between proximal and more distal synapses (Andrasfalvy and Magee, 2001; Williams and Stuart, 2002; Smith et al, 2003). However, distal inputs being further away, they may be influenced to a smaller degree by back-propagating action potentials that can contribute to synaptic plasticity in the BLA (Humeau et al, 2005). In many regions, there is a topographic arrangement of excitatory synaptic inputs to dendritic subregions (Steward, 1976; Amaral and Witter, 1989; Markram et al, 1997; French and Totterdell, 2002; Bollmann and Engert, 2009; Triplett et al, 2009; Jia et al, 2010). However, it is unknown whether a topography exists in the distribution of excitatory inputs to BA neurons. This impedes the ability to draw conclusions about whether a specific set of inputs to the BA are altered by chronic stress. Future studies that determine the source of the new synaptic inputs and their impact may be able to explore their relative strength and plasticity.
It is possible that the increased frequency of EPSPs is unrelated to the increased number of spines. Numerous factors can increase the release of glutamate in the BA, including stress-related transmitters and hormones, such as norepinepherine and corticosterone (DeBock et al, 2003; Karst et al, 2010). The impact of both of these factors on BA neuronal activity will likely depend on the history of stress (Braga et al, 2004; Buffalari and Grace, 2009; Karst et al, 2010). A different alternative possibility is that the new spines are functionally inactive, and an increase of network activity underlies the increased frequency of spontaneous EPSPs, or the increased synaptic events may originate from dendritic shafts, not spines. Several observations make this a less likely explanation. First, we observed a positive correlation between the effects of repeated stress on spine number and the rise time of synaptic events. This association would not be expected unless the spine number and synaptic events were changing together. In addition, there is little evidence for network changes that would underlie the effects of repeated stress on EPSP frequency. For example, there was no significant change in the frequency of clusters of synaptic events. Furthermore, the anesthesia state that strongly influences network activity was monitored by EEG, and kept within a relatively narrow range.
These findings are consistent with previous studies that have found an increase in the number of spines in BA neurons after repeated stressors. Previous studies did not test whether the increase of spines was global or more limited. Furthermore, it was assumed that the increased spines translated into an increased excitatory drive, which can be a problematic assumption, given the existance of silent or functionally weak synapses. This study demonstrates that the increase of spines occurs at intermediate and distal distances from the soma, and it also demonstrates that the increased spines impart a functional in vivo impact on these neurons. The importance of these findings is that they suggest a shift in the source and shape of the drive of BA neurons after repeated stress. In addition, the greater number of functional synapses may cause the higher spontaneous firing rates and responsiveness of BA neurons after repeated stress. Furthermore, it points to the possibility of disproportionate enhancement of a specific set of excitatory synaptic inputs that may now excessively drive BA neurons.
An increase of synaptic drive of BA neurons is likely to increase the neuronal output. By increasing neuronal output, the BA will contribute more readily to ongoing behaviors. The impact of increased BA output may be complex, given the predominantly inhibitory impact on neurons of the central amygdala (Collins and Pare, 1999; Rosenkranz et al, 2006), but may result in disinhibition of a proportion of neurons in the medial division of the CeA (Haubensak et al, 2010; Duvarci et al, 2011; Amano et al, 2012), allowing a range of affective behaviors. This may account for an increase in behaviors that are modified by the BLA, such as anxiety-like behaviors and increased fear conditioning observed after repeated stress (Conrad et al, 1999; Vyas and Chattarji, 2004; Wood et al, 2008; Atchley et al, 2012). These morphological and functional changes may underlie amygdala hyperactivity, and in combination with effects in the prefrontal cortex, hippocampus, and nucleus accumbens (Magarinos et al, 1996; Shors et al, 2001; Radley et al, 2008; Lei and Tejani-Butt, 2010; Christoffel et al, 2011; Muhammad et al, 2012) account for the ability of chronic stress to exacerbate affective disorders (Heim and Nemeroff, 2001; Lupien et al, 2009).
References
Adamec R, Blundell J, Burton P (2005). Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav 86: 75–91.
Adamec R, Hebert M, Blundell J, Mervis RF (2012). Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav Brain Res 226: 133–146.
Amano T, Amir A, Goswami S, Pare D (2012). Morphology, PKCdelta expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons. J Neurophysiol 108: 3196–3205.
Amaral DG, Witter MP (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591.
Andrasfalvy BK, Magee JC (2001). Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci 21: 9151–9159.
Andreasen M, Lambert JD (1998). Factors determining the efficacy of distal excitatory synapses in rat hippocampal CA1 pyramidal neurones. J Physiol 507: 441–462.
Armony JL, Corbo V, Clement MH, Brunet A (2005). Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry 162: 1961–1963.
Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA (2012). Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res 232: 37–43.
Baer SM, Rinzel J (1991). Propagation of dendritic spikes mediated by excitable spines: a continuum theory. J Neurophysiol 65: 874–890.
Bhatnagar S, Dallman M (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84: 1025–1039.
Bogdan R, Williamson DE, Hariri AR (2012). Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry 169: 515–522.
Bollmann JH, Engert F (2009). Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron 61: 895–905.
Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H (2004). Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology 29: 45–58.
Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN et al (1996). Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 53: 595–606.
Buffalari DM, Grace AA (2007). Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci 27: 12358–12366.
Buffalari DM, Grace AA (2009). Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol 12: 95–107.
Chen FJ, Sara SJ (2007). Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience 144: 472–481.
Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF et al (2011). IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31: 314–321.
Collins DR, Pare D (1999). Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci 19: 836–844.
Conrad CD, LeDoux JE, Magarinos AM, McEwen BS (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113: 902–913.
Contreras D, Steriade M (1995). Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 15: 604–622.
Correll CM, Rosenkranz JA, Grace AA (2005). Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry 58: 382–391.
Crane JW, Windels F, Sah P (2009). Oscillations in the basolateral amygdala: aversive stimulation is state dependent and resets the oscillatory phase. J Neurophysiol 102: 1379–1387.
Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71: 286–293.
Davidson RJ, Irwin W, Anderle MJ, Kalin NH (2003). The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160: 64–75.
DeBock F, Kurz J, Azad SC, Parsons CG, Hapfelmeier G, Zieglgänsberger W et al (2003). Alpha2-adrenoreceptor activation inhibits LTP and LTD in the basolateral amygdala: involvement of Gi/o-protein-mediated modulation of Ca2+-channels and inwardly rectifying K+-channels in LTD. Eur J Neurosci 17: 1411–1424.
Destexhe A, Pare D (1999). Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81: 1531–1547.
Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME (1992). A functional anatomical study of unipolar depression. J Neurosci 12: 3628–3641.
Duvarci S, Popa D, Pare D (2011). Central amygdala activity during fear conditioning. J Neurosci 31: 289–294.
Farb C, Aoki C, Milner T, Kaneko T, LeDoux J (1992). Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res 593: 145–158.
French SJ, Totterdell S (2002). Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446: 151–165.
Ganzel B, Casey BJ, Glover G, Voss HU, Temple E (2007). The aftermath of 9/11: effect of intensity and recency of trauma on outcome. Emotion 7: 227–238.
Garcia R, Paquereau J, Vouimba RM, Jaffard R (1998). Footshock stress but not contextual fear conditioning induces long-term enhancement of auditory-evoked potentials in the basolateral amygdala of the freely behaving rat. Eur J Neurosci 10: 457–463.
Gaudreau H, Pare D (1996). Projection neurons of the lateral amygdaloid nucleus are virtually silent throughout the sleep–waking cycle. J Neurophysiol 75: 1301–1305.
Haider B, Duque A, Hasenstaub AR, McCormick DA (2006). Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276.
Heim C, Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49: 1023–1039.
Hill MN, Hillard CJ, McEwen BS (2011). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex 21: 2056–2064.
Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM et al (2012). Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry doi:10.1038/mp.2012.90.
Humeau Y, Herry C, Kemp N, Shaban H, Fourcaudot E, Bissière S et al (2005). Dendritic spine heterogeneity determines afferent-specific Hebbian plasticity in the amygdala. Neuron 45: 119–131.
Isoardi NA, Bertotto ME, Martijena ID, Molina VA, Carrer HF (2007). Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci 26: 1036–1044.
Jaffe DB, Carnevale NT (1999). Passive normalization of synaptic integration influenced by dendritic architecture. J Neurophysiol 82: 3268–3285.
Jaslove SW (1992). The integrative properties of spiny distal dendrites. Neuroscience 47: 495–519.
Jia H, Rochefort NL, Chen X, Konnerth A (2010). Dendritic organization of sensory input to cortical neurons in vivo. Nature 464: 1307–1312.
Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL (1985). Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav 22: 631–634.
Karst H, Berger S, Erdmann G, Schutz G, Joels M (2010). Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 107: 14449–14454.
Kavushansky A, Richter-Levin G (2006). Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res 84: 1580–1587.
Kavushansky A, Vouimba RM, Cohen H, Richter-Levin G (2006). Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus 16: 35–42.
Lampl I, Reichova I, Ferster D (1999). Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22: 361–374.
Lang EJ, Pare D (1997). Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol 77: 341–352.
Larkum ME, Senn W, Luscher HR (2004). Top–down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex 14: 1059–1070.
Lei Y, Tejani-Butt SM (2010). N-methyl-D-aspartic acid receptors are altered by stress and alcohol in Wistar–Kyoto rat brain. Neuroscience 169: 125–131.
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10: 434–445.
Machne X, Segundo JP (1956). Unitary responses to afferent volleys in amygdaloid complex. J Neurophysiol 19: 232–240.
Magarinos AM, McEwen BS, Flugge G, Fuchs E (1996). Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16: 3534–3540.
Magee JC (2000). Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 1: 181–190.
Magee JC, Cook EP (2000). Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3: 895–903.
Mainen ZF, Sejnowski TJ (1996). Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382: 363–366.
Markram H, Lubke J, Frotscher M, Roth A, Sakmann B (1997). Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol 500: 409–440.
McDonald AJ (1982). Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol 212: 293–312.
McDonald AJ, Muller JF, Mascagni F (2002). GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol 446: 199–218.
Mikics E, Tóth M, Varjú P, Gereben B, Liposits Z, Ashaber M et al (2008). Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology 33: 1198–1210.
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA 102: 9371–9376.
Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S et al (2010). Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci 30: 5357–5367.
Muhammad A, Carroll C, Kolb B (2012). Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 216: 103–109.
Muller JF, Mascagni F, McDonald AJ (2006). Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin–immunoreactive interneurons. J Comp Neurol 494: 635–650.
Muller JF, Mascagni F, McDonald AJ (2007). Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol 500: 513–529.
Muramoto K, Ono T, Nishijo H, Fukuda M (1993). Rat amygdaloid neuron responses during auditory discrimination. Neuroscience 52: 621–636.
Oviedo H, Reyes AD (2005). Variation of input–output properties along the somatodendritic axis of pyramidal neurons. J Neurosci 25: 4985–4995.
Pare D, Dong J, Gaudreau H (1995a). Amygdalo-entorhinal relations and their reflection in the hippocampal formation: generation of sharp sleep potentials. J Neurosci 15: 2482–2503.
Pare D, Pape HC, Dong J (1995b). Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol 74: 1179–1191.
Paxinos G, Watson C (1998). The rat brain in stereotaxic coordinates, spiral bound. Academic Press: New York.
Pelletier JG, Likhtik E, Filali M, Pare D (2005). Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn Mem 12: 96–102.
Petersen CC, Grinvald A, Sakmann B (2003). Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured – by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23: 1298–1309.
Poulet JF, Petersen CC (2008). Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454: 881–885.
Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W et al (2005). Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry 57: 464–473.
Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE (2010). Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci 30: 4676–4686.
Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR et al (2007). Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res 1134: 87–94.
Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS et al (2008). Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507: 1141–1150.
Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA (2005). Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci 25: 8725–8734.
Romanski LM, Clugnet MC, Bordi F, LeDoux JE (1993). Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci 107: 444–450.
Rosenkranz JA (2011). Neuronal activity causes rapid changes of lateral amygdala neuronal membrane properties and reduction of synaptic integration and synaptic plasticity in vivo. J Neurosci 31: 6108–6120.
Rosenkranz JA (2012). In vivo voltage-dependent influences on summation of synaptic potentials in neurons of the lateral nucleus of the amygdala. Neuroscience 226: 101–118.
Rosenkranz JA, Buffalari DM, Grace AA (2006). Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biol Psychiatry 59: 801–811.
Rosenkranz JA, Grace AA (2002). Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22: 324–337.
Rosenkranz JA, Johnston D (2007). State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex. J Neurosci 27: 7054–7069.
Rosenkranz JA, Venheim ER, Padival M (2010). Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 67: 1128–1136.
Sanchez-Vives MV, McCormick DA (2000). Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034.
Schaefer AT, Larkum ME, Sakmann B, Roth A (2003). Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol 89: 3143–3154.
Segev I, Rall W (1988). Computational study of an excitable dendritic spine. J Neurophysiol 60: 499–523.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50: 651–658.
Shepherd GM, Brayton RK, Miller JP, Segev I, Rinzel J, Rall W (1985). Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proc Natl Acad Sci USA 82: 2192–2195.
Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL et al (1997). Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry 54: 233–241.
Shors TJ (1999). Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport 10: 2811–2815.
Shors TJ, Chua C, Falduto J (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21: 6292–6297.
Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002). Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51: 693–707.
Smith MA, Ellis-Davies GC, Magee JC (2003). Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol 548: 245–258.
Stamp JA, Herbert J (1999). Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience 94: 1313–1322.
Steriade M, McCormick DA, Sejnowski TJ (1993a). Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685.
Steriade M, Nunez A, Amzica F (1993b). Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13: 3266–3283.
Steward O (1976). Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167: 285–314.
Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH et al (2001). Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 58: 1057–1063.
Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP et al (2009). Retinal input instructs alignment of visual topographic maps. Cell 139: 175–185.
Turner DA (1988). Waveform and amplitude characteristics of evoked responses to dendritic stimulation of CA1 guinea-pig pyramidal cells. J Physiol 395: 419–439.
van Wingen GA, Geuze E, Vermetten E, Fernandez G (2011). Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry 16: 664–671.
van Wingen GA, Geuze E, Vermetten E, Fernandez G (2012). The neural consequences of combat stress: long-term follow-up. Mol Psychiatry 17: 116–118.
Vetter P, Roth A, Hausser M (2001). Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol 85: 926–937.
Volgushev M, Chauvette S, Mukovski M, Timofeev I (2006). Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected]. J Neurosci 26: 5665–5672.
Vouimba RM, Munoz C, Diamond DM (2006). Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress 9: 29–40.
Vouimba RM, Yaniv D, Diamond D, Richter-Levin G (2004). Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur J Neurosci 19: 1887–1894.
Vyas A, Chattarji S (2004). Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci 118: 1450–1454.
Vyas A, Jadhav S, Chattarji S (2006). Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–393.
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818.
Vyas A, Pillai AG, Chattarji S (2004). Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128: 667–673.
Williams SR (2005). Encoding and decoding of dendritic excitation during active states in pyramidal neurons. J Neurosci 25: 5894–5902.
Williams SR, Stuart GJ (2002). Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295: 1907–1910.
Windels F, Crane JW, Sah P (2010). Inhibition dominates the early phase of up-states in the basolateral amygdala. J Neurophysiol 104: 3433–3438.
Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS (2008). Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci 122: 282–292.
Zhang W, Rosenkranz JA (2012). Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience 226: 459–474.
Acknowledgements
We thank Mitch Beales for initial histological help and Dr Janice Urban and Dr Kuei Tseng for advice and suggestions. A portion of these data was previously presented at the Society for Neuroscience annual meeting. This work was supported by NIH (MH084970) and The Brain Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Rosenkranz received funding from NIH and The Brain research Foundation for these studies. These funding agencies had no role in the design of the study, collection, and analysis of data or decision to publish. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Padival, M., Quinette, D. & Rosenkranz, J. Effects of Repeated Stress on Excitatory Drive of Basal Amygdala Neurons In Vivo. Neuropsychopharmacol 38, 1748–1762 (2013). https://doi.org/10.1038/npp.2013.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.74