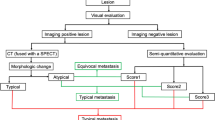
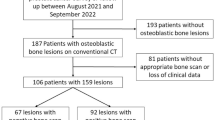
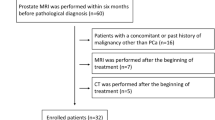
Abstract
Bone metastases of solid tumors are common, and about 80% of them occur in patients with breast, lung or prostate cancer. Bone metastases can be suspected clinically and by laboratory tests; however, a final diagnosis relies on radiographic evidence. Bone metastases of prostate cancer usually have osteoblastic characteristics, manifested by pathological bone resorption and formation. Conventional bone scans (e.g. with 99mTc-labeled methylene diphosphonate) are preferred to plain-film radiography for surveillance of the entire skeleton. Radiologic diagnosis of bone metastases, particularly in patients with low burden of disease, is difficult because noncancerous bone lesions that mimic cancer are common. Conventional bone scans are limited by their low sensitivity and high false-negative rate (up to 40%) compared with advanced bone-imaging modalities such as PET, PET–CT and MRI, which might assist or replace conventional scanning methods. The correct diagnosis of bone involvement in prostate cancer is crucial to assess the effects of therapy on the primary tumor, the patient's prognosis, and the efficacy of bone-specific treatments that can reduce future bone-associated morbidity. In addition, predictive tools such as nomograms enable the identification of patients at risk of bone involvement during the course of their disease. Such tools may limit treatment costs by avoidance of unnecessary tests and might reduce both short-term and long-term complication rates.
Key Points
-
Bone metastases are common in patients with advanced prostate cancer, and diagnosis of such metastases currently relies on imaging studies
-
The most commonly used bone-imaging modality in prostate cancer is the radionuclide bone scan, because this technique is easily available and inexpensive; however, radionuclide bone scans have limited sensitivity and specificity
-
Modern imaging modalities such as PET, PET–CT and MRI have improved accuracy in the detection of bone metastases of prostate cancer; however, these techniques have major limitations including high cost and lack of availability
-
Staging of newly diagnosed prostate cancer is usually done according to the patient's PSA level at diagnosis, digital rectal examination and biopsy results, which considerably reduces the number of tests performed
-
The prevalence of bone involvement in patients who have rising PSA levels after radical prostatectomy is associated with several clinical variables, the most important of which are dynamic changes in PSA (PSA doubling time and PSA velocity); these variables were used to construct a nomogram used to predict the probability of bone involvement at any specific time point, to determine whether patients require bone imaging, and to select patients for clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 15: 1588–1594
Berenson JR et al. (2006) Pathophysiology of bone metastases. Cancer Biol Ther 5: 1078–1081
Jemal A et al. (2007) Cancer statistics 2007. CA Cancer J Clin 57: 43–66
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593
Reikerås O et al. (1988) Bone scintigraphy, radiographic survey and prostatic acid phosphatase in patients with prostatic carcinoma. A comparison of sensitivity. Int Urol Nephrol 20: 51–54
Rosenthal DI (1997) Radiologic diagnosis of bone metastases. Cancer 15: 1595–1607
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350: 1655–1656
Raubenheimer EJ et al. (2006) Pathogenesis of bone metastasis. J Oral Pathol Med 35: 129–135
Keller ET et al. (2001) Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev 20: 333–349
Selvaggi G et al. (2005) Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol 56: 365–378
Scott LJ et al. (2001) Interactions of human prostatic epithelial cells with bone marrow endothelium: binding and invasion. Br J Cancer 84: 1417–1423
Choueiri MB et al (2006) The central role of osteoblasts in the metastasis of prostate cancer. Cancer Metastasis Rev 25: 601–609
Lehr JE and Pienta KJ (1998) Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst 90: 118–123
Subramanian G et al. (1972) 99Tc-labeled polyphosphate as a skeletal imaging agent. Radiology 102: 701–704
Osmond JD et al. (1975) Accuracy of 99mTc-diphosphonate bone scans and roentgenograms in the detection of prostate, breast and lung carcinoma metastases. Am J Roentgenol 125: 972–977
Basu S et al. (2004) 99mTcVDMSA scintigraphy in skeletal metastases and superscans arising from various malignancies: diagnosis, treatment monitoring and therapeutic implications. Br J Radiol 77: 347–361
Savelli G et al. (2001) Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. QJ Nucl Med 45: 27–37
Even-Sapir E et al. (2006) The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 47: 287–297
Kattan MW et al. (1998) A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 90: 766–771
D'Amico AV et al. (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280: 969–974
Pound CR et al. (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281: 1591–1597
Roberts SG et al. (2001) PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc 76: 576–581
Freedland SJ et al. (2005) Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 294: 433–439
Dotan ZA et al. (2005) Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol 23: 1962–1968
Crawford ED et al. (1989) A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321: 419–424
Knudson G et al. (1991) Bone scan as a stratification variable in advanced prostate cancer. Cancer 68: 316–320
Soloway MS et al. (1988) Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61: 195–202
Rigaud J et al. (2002) Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol 168: 1423–1426
Cook GJ and Fogelman I (2001) The role of nuclear medicine in monitoring treatment in skeletal malignancy. Semin Nucl Med 31: 206–211
Vogel CL et al. (1995) Worsening bone scan in the evaluation of antitumor response during hormonal therapy of breast cancer. J Clin Oncol 13: 1123–1128
Johns WD et al. (1990) Leuprolide therapy for prostate cancer. An association with scintigraphic “flare” on bone scan. Clin Nucl Med 15: 485–487
Imbriaco M et al. (1998) A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res 4: 1765–1772
Sabbatini P et al. (1999) Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol 17: 948–957
Noguchi M et al. (2003) Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer 27: 195–201
Yahara J et al. (2003) Quantitative evaluation of bone metastases in patients with advanced prostate cancer during systemic treatment. BJU Int 92: 379–383
Hricak H et al. (2007) Imaging prostate cancer: a multidisciplinary perspective. Radiology 243: 28–53
Brenner DJ et al. (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357: 2277–2284
Sanal SM et al. (1994) Detection of bone marrow involvement in breast cancer with magnetic resonance imaging. J Clin Oncol 12: 1415–1421
Steinborn MM et al. (1999) Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr 23: 123–129
Traill ZC et al. (1999) Magnetic resonance imaging versus radionuclide scintigraphy in screening for bone metastases. Clin Radiol 54: 448–451
Bayley A et al. (2001) A prospective study of factors predicting clinically occult spinal cord compression in patients with metastatic prostate carcinoma. Cancer 92: 303–310
Lecouvet FE et al. (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25: 3281–3287
Juweid ME et al. (2006) Positron-emission tomography and assessment of cancer therapy. N Engl J Med 354: 496–507
Schöder H et al. (2005) CT in PET/CT: essential features of interpretation. J Nucl Med 46: 1249–1251
Bar-Shalom R et al. (2003) Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 44: 1200–1209
Taira AV et al. (2007) Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology 243: 204–211
Schöder H et al. (2005) 2-18F-fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 11: 4761–4769
Liu IJ et al. (2001) Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 7: 108–111
Morris MJ et al. (2005) Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res 11: 7454–7461
Larson SM et al. (2008) Advances in positron emission tomography applications for urologic cancers. Curr Opin Urol 18: 65–70
Oyama N et al. (2003) 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med 44: 549–555
Rinnab L et al. (2007) Evaluation of 11C-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int 100: 786–793
Gutman F et al. (2006) 18F-choline PET/CT for initial staging of advanced prostate cancer. AJR Am J Roentgenol 187: W618–W621
NCCN practice guidelines in oncology—prostate cancer 2007 edition. [http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf] (accessed online 30 June 2008)
Cooperberg MR et al. (2005) The changing face of prostate cancer. J Clin Oncol 23: 8146–8151
Partin AW et al. (1997) Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. JAMA 277: 1445–1451
Stephenson AJ et al. (2006) Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 98: 715–717
Abuzallouf S et al. (2004) Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 171: 2122–2127
Leibovici D et al. (2007) Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 15: 198–204
Wolff JM et al. (1998) Is prostate-specific antigen a reliable marker of bone metastasis in patients with newly diagnosed cancer of the prostate? Eur Urol 33: 376–381
Lorente JA et al. (1999) Serum bone alkaline phosphatase levels enhance the clinical utility of prostate-specific antigen in the staging of newly diagnosed prostate cancer patients. Eur J Nucl Med 26: 625–632
Shreve PD et al. (1996) Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-18F-fluoro-[D]-glucose. Radiology 199: 751–756
Salminen E et al. (2002) Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol 41: 425–429
Picchio M et al. (2003) Value of 11C-choline-positron emission tomography for re-staging prostate cancer: a comparison with 18F-fluorodeoxyglucose-positron emission tomography. J Urol 169: 1337–1340
Langsteger W et al. (2006) The role of fluorodeoxyglucose, 18F-dihydroxyphenylalanine, 18F-choline, and 18F-fluoride in bone imaging with emphasis on prostate and breast. Semin Nucl Med 36: 73–92
Heidenreich A et al. Guidelines on prostate cancer. © European Urological Association 2007 [http://www.uroweb.org/fileadmin/user_upload/Guidelines/ 07_Prostate_Cancer_2007.pdf] (accessed 30 June 2008)
Guidelines for the management of clinically localized prostate cancer: 2007 update. American Urological Association [http://www.auanet.org/guidelines/main_reports/proscan07/content.pdf] (accessed 30 June 2008)
Bill-Axelson A et al. (2005) Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 352: 1977–1984
Nagda SN et al. (2007) Long-term follow-up of 111In-capromab pendetide scan as pretreatment assessment in patients who undergo salvage radiotherapy for rising prostate-specific antigen after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys 67: 834–840
Terris MK et al. (1991) Utilization of bone scans in conjunction with prostate-specific antigen levels in surveillance for recurrence of adenocarcinoma after radical prostatectomy. J Nucl Med 32: 1713–1717
Cher ML et al. (1998) Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol 160: 1387–1391
Kane CJ et al. (2003) Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 61: 607–611
Stephenson AJ et al. (2007) Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 25: 2035–2041
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Dotan, Z. Bone imaging in prostate cancer. Nat Rev Urol 5, 434–444 (2008). https://doi.org/10.1038/ncpuro1190
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro1190
This article is cited by
-
PET and PET/CT with radiolabeled choline in prostate cancer: a critical reappraisal of 20 years of clinical studies
European Journal of Nuclear Medicine and Molecular Imaging (2017)
-
Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: analysis of three clinical trials
British Journal of Cancer (2012)
-
Advances in multimodality molecular imaging of bone structure and function
BoneKEy Reports (2012)
-
Role of 11C-choline positron emission tomography/computed tomography in evaluating patients affected by prostate cancer with suspected relapse due to prostate-specific antigen elevation
Japanese Journal of Radiology (2011)
-
Whole-body MRI (WB-MRI) versus axial skeleton MRI (AS-MRI) to detect and measure bone metastases in prostate cancer (PCa)
European Radiology (2010)