Abstract
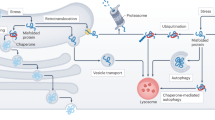
Rheumatoid arthritis is a disease associated with painful joints that affects approximately 1% of the population worldwide, and for which no specific cure is available. Among other functions, the endoplasmic reticulum (ER) has an important role in protein folding. When the level of unfolded proteins in the ER exceeds the folding capacity of this organelle, defective proteins are eliminated by ER-associated degradation (ERAD), an ATP-dependent ubiquitin–proteasome degradation process, to reduce the burden on the ER. Synoviolin is an E3 ubiquitin ligase that is involved in ERAD. This protein is a pathogenic factor for arthropathy; it is overexpressed in the synovial cells of patients with rheumatoid arthritis. This overexpression results in a 'hyper-ERAD' state, in which the cell deals with accumulated unfolded proteins excessively. Rheumatoid synovial cells produce large amounts of various proteins such as cytokines and proteases, which consequently might confer an autonomous proliferation property on the cells. At least 30% of all newly synthesized, ER-sorted proteins are unfolded. Although degradation of unfolded proteins consumes large amounts of ATP and would seem an unconventional process, it is essential for joint homeostasis.
Key Points
-
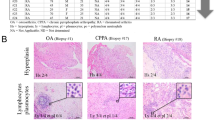
Synoviolin, an E3 ubiquitin ligase that is associated with endoplasmic reticulum-associated degradation (ERAD), is highly expressed in rheumatoid synovial cells and is involved in the onset of rheumatoid arthritis (RA)
-
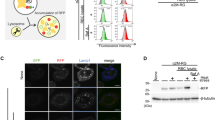
Synoviolin targets the tumor suppressor protein p53 for proteasomal degradation, and can regulate both ER-stress-induced and p53-dependent apoptotic pathways
-
Rheumatoid synovial cells undergo autonomous proliferation and aberrant protein production caused by hyper-ERAD by overexpression of synoviolin
-
Approximately 30% of newly synthesized proteins are unfolded; the cell utilizes ATP to degrade these proteins, which enables synovial cells to maintain a single-layer structure
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423: 356–361
Arend WP (2001) Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum 45: 101–106
McInnes IB and Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7: 429–442
Stanczyk J et al. (2006) Synovial cell activation. Curr Opin Rheumatol 18: 262–267
Huber LC et al. (2006) Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 45: 669–675
Knedla A et al. (2007) Developments in the synovial biology field 2006. Arthritis Res Ther 9: 209
Spencer-Green G (2000) Etanercept (Enbrel): update on therapeutic use. Ann Rheum Dis 59 (Suppl 1): i46–49
St Clair EW (2002) Infliximab treatment for rheumatic disease: clinical and radiological efficacy. Ann Rheum Dis 61 (Suppl 2): ii67–69
Amano T et al. (2003) Synoviolin, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev 17: 2436–2449
Kaneko M et al. (2002) Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation. FEBS Lett 532: 147–152
Nadav E et al. (2003) A novel mammalian endoplasmic reticulum ubiquitin ligase homologous to the yeast Hrd1. Biochem Biophys Res Commun 303: 91–97
Kikkert M et al. (2004) Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem 279: 3525–3534
Bordallo J et al. (1998) Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell 9: 209–222
Shearer AG and Hampton RY (2004) Structural control of endoplasmic reticulum-associated degradation: effect of chemical chaperones on 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem 279: 188–196
Yagishita N et al. (2005) Essential role of synoviolin in embryogenesis. J Biol Chem 280: 7909–7916
Shimaoka Y et al. (1998) Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest 102: 606–618
Tomita T et al. (1999) Establishment of nurse-like stromal cells from bone marrow of patients with rheumatoid arthritis: indication of characteristic bone marrow microenvironment in patients with rheumatoid arthritis. Rheumatology 38: 854–863
Yagishita N et al.: What do synoviolin deficient mice tell us? In Bone Marrow in RA Patients (Eds Ochi T and Lipsky PE), in press
Yamasaki S et al. (2007) Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase 'Synoviolin'. EMBO J 26: 113–122
Haupt Y et al. (1997) Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299
Kubbutat MH et al. (1997) Regulation of p53 stability by Mdm2. Nature 387: 299–303
Leng RP et al. (2003) Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112: 779–791
Dornan D et al. (2004) The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429: 86–92
Firestein GS et al. (1997) Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA 94: 10895–10900
Reme T et al. (1998) Mutations of the p53 tumour suppressor gene in erosive rheumatoid synovial tissue. Clin Exp Immunol 111: 353–358
Inazuka M et al. (2000) Analysis of p53 tumour suppressor gene somatic mutations in rheumatoid arthritis synovium. Rheumatology 39: 262–266
Muller-Ladner U and Nishioka K (2000) p53 in rheumatoid arthritis: friend or foe? Arthritis Res 2: 175–178
Sun Y and Cheung HS (2002) p53, proto-oncogene and rheumatoid arthritis. Semin Arthritis Rheum 31: 299–310
Yamanishi Y et al. (2002) Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol 160: 123–130
Simelyte E et al. (2005) Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum 52: 1876–1884
Ellgaard L et al. (1999) Setting the standards: quality control in the secretory pathway. Science 286: 1882–1888
Schroder M and Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789
Rutkowski DT and Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28
Yamasaki S et al. (2005) Rheumatoid arthritis as a hyper-endoplasmic-reticulum-associated degradation disease. Arthritis Res Ther 7: 181–186
Yagishita N et al. (2006) Role of Synoviolin in rheumatoid arthritis: possible clinical relevance. Future Rheumatol 1: 31–36
Ritchlin C (2000) Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res 2: 356–360
Yamasaki S et al. (2006) Resistance to endoplasmic reticulum stress is an acquired cellular characteristic of rheumatoid synovial cells. Int J Mol Med 18: 113–117
Zhao L and Ackerman SL (2006) Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18: 444–452
Yoshida H (2007) ER stress and diseases. FEBS J 274: 630–658
Bence NF et al. (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292: 1552–1555
Hirabayashi M et al. (2001) VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ 8: 977–984
Jana NR et al. (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10: 1049–1059
Imai Y et al. (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275: 35661–35664
Vabulas RM et al. (2005) Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 23: 1960–1963
Yewdell JW (2001) Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol 11: 294–297
Hershko A and Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479
Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533
International Human Genome Sequencing Consortium (2004) Finishing the euchromatic sequence of the human genome. Nature 431: 931–945
Toh ML et al. (2006) Overexpression of synoviolin in peripheral blood and synoviocytes from rheumatoid arthritis patients and continued elevation in nonresponders to infliximab treatment. Arthritis Rheum 54: 2109–2118
Tsuchimochi K et al. (2005) Identification of a crucial site for synoviolin expression. Mol Cell Biol 25: 7344–7356
Gao B et al. (2006) The proinflammatory cytokines IL-1beta and TNF-alpha induce the expression of Synoviolin, an E3 ubiquitin ligase, in mouse synovial fibroblasts via the Erk1/2-ETS1 pathway. Arthritis Res Ther 8: R172
Acknowledgements
We thank N Okamoto, Fun-site, ITAKURA OFFICE and all members of Professor Nakajima's laboratory. This work was partially supported financially by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Creative research conducted by university researchers individually or in groups Category A, Category C), Ministry of Health, Labour and Welfare Grants-in-Aid for Scientific Research (Research on Allergic disease and Immunology), the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (04-3), the Kanagawa Nanbyo Foundation, Heiwa Nakajima Foundation, The Uehara Memorial Foundation, Takeda Science Foundation, Mitsui Life Insurance Co. Ltd. and the Sagawa Foundation for Promotion of Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yagishita, N., Yamasaki, S., Nishioka, K. et al. Synoviolin, protein folding and the maintenance of joint homeostasis. Nat Rev Rheumatol 4, 91–97 (2008). https://doi.org/10.1038/ncprheum0699
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0699
This article is cited by
-
Synoviolin meets metabolic disorders
Arthritis Research & Therapy (2012)
-
The functions of the post-translational modifications in rheumatoid arthritis
Arthritis Research & Therapy (2012)
-
The contribution of Asian researchers to the field of rheumatology
Nature Reviews Rheumatology (2010)