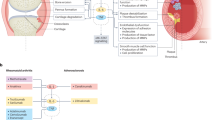
Abstract
Advances in molecular biology and the clinical success of strategies that target tumor necrosis factor (TNF) have led to further research into the pathophysiology of human rheumatoid arthritis. Several novel therapeutic targets have emerged from these efforts, including not only molecules that regulate TNF (e.g. TNF-α converting enzyme), the complex cytokine network (e.g. interleukin [IL]-6, IL-15, IL-17) and several adipokines, but also targets that originate from cellular and subcellular components of the disease. Strategies that aim at cellular targets include antibodies to CD20 or BLyS (also known as TNF ligand family member 13b), which deplete or inhibit B cells, as well as approaches that interfere with membrane-derived microparticles. Components of subcellular pathways, which are predominantly upstream of the central regulator of transcription nuclear factor κB, have also been studied. Of these, strategies that target mitogen-activated protein kinases have a leading role and are on the verge of clinical use; approaches that target specific molecules such as Janus kinases, signal transducer and activator of transcription proteins, and suppressor of cytokine signaling proteins also seem to show promise and might have a clinical application in the future.
Key Points
-
Biologics are a novel class of agents that are designed to target defined pathogenetic mechanisms and molecules with high specificity
-
Interleukin (IL)-6, IL-15, IL-17 and receptor activator of nuclear factor κB ligand form a complex cytokine network with IL-1 and tumor necrosis factor that promotes joint inflammation and joint destruction
-
Adipokines and microparticles are intriguing targets for new biologics
-
The mitogen-activated protein kinases and transcription factors such as nuclear factor κB are essential intracellular mediators of inflammation and, therefore, are potentially powerful targets, but must be selected carefully because of possible adverse effects
-
Components of intracellular signaling pathways upstream of nuclear factor κB seem to be safer, although potentially less effective, than downstream targets
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Furst DE et al. (2006) Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2006. Ann Rheum Dis 65 (Suppl 3): iii2–iii15
Pollard L and Choy E (2005) Rheumatoid arthritis: non-tumor necrosis factor targets. Curr Opin Rheumatol 17: 242–246
Study to assess the effect of tocilizumab plus methotrexate on signs and symptoms in patients with moderate to severe active rheumatoid arthritis [http://www.clinicaltrials.gov/ct/show/NCT00106548?order=1] (accessed 28 March 2007)
Choy EH et al. (2002) Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 46: 3143–3150
McInnes IB et al. (2003) New strategies to control inflammatory synovitis: interleukin 15 and beyond. Ann Rheum Dis 62 (Suppl 2): ii51–ii54
Ferrari-Lacraz S et al. (2004) Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15–Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol 173: 5818–5826
Miranda-Carús ME et al. (2004) IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lympocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol 173: 1463–1476
Ruchatz H et al. (1998) Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol 160: 5654–5660
Baslund B et al. (2005) Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum 52: 2686–2692
McKenzie BS et al. (2006) Understanding the IL-23–IL-17 immune pathway. Trends Immunol 27: 17–23
Kim HR et al. (2007) Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-κB- and p38 MAPK-dependent signaling pathways. Rheumatology 46: 57–64
Hwang SY et al. (2004) IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-κB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther 6: R120–R128
Lubberts E (2003) The role of IL-17 and family members in the pathogenesis of arthritis. Curr Opin Investig Drugs 4: 572–577
Lubberts E et al. (2005) The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther 7: 29–37
Lubberts E et al. (2004) Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 50: 650–659
Schett G et al. (2005) Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 1: 47–54
Neumann E et al. (2005) The RANK/RANKL/osteoprotegerin system in rheumatoid arthritis: new insights from animal models. Arthritis Rheum 52: 2960–2967
Geusens PP et al. (2006) The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum 54: 1772–1777
Bekker PJ et al. (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16: 348–360
Bekker PJ et al. (2004) A single-dose placebo controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059–1066
Edwards JC et al. (2006) B cell depletion therapy in rheumatic disease. Best Pract Res Clin Rheumatol 20: 915–928
Cheema GS et al. (2001) Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 44: 1313–1319
Seyler TM et al. (2005) BLyS and APRIL in rheumatoid arthritis. J Clin Invest 115: 1083–1092
Ohata J et al. (2005) Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol 174: 864–870
Wang H et al. (2001) TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol 2: 632–637
Gross JA et al. (2001) TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: impaired B cell maturation in mice lacking BLyS. Immunity 15: 289–302
Ding C and Jones G (2006) Belimumab Human Genome Sciences/Cambridge Antibody Technology/GlaxoSmithKline. Curr Opin Investig Drugs 7: 464–472
Cohen SB et al. (2006) Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54: 2793–2806
Roschke V et al. (2002) BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol 169: 4314–4321
Wagner G and Laufer S (2006) Small molecular anti-cytokine agents. Med Res Rev 26: 1–62
Conway JG et al. (2001) Inhibition of tumor necrosis factor-α (TNF-α) production and arthritis in the rat by GW3333, a dual inhibitor of TNF-α-converting enzyme and matrix metalloproteinases. J Pharmacol Exp Ther 298: 900–908
Schäffler A et al. (2006) Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev 27: 449–467
Neumeier M et al. (2006) Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol 79: 803–808
Weigert J et al. (2005) The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett 579: 5565–5570
Otero M et al. (2006) Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology 45: 944–950
Otero M et al. (2006) Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis 65: 1198–1201
Schäffler A et al. (2003) Adipocytokines in synovial fluid. JAMA 290: 1709–1710
Ehling A et al. (2006) The potential of adiponectin in driving arthritis. J Immunol 176: 4468–4478
Busso N et al. (2002) Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 168: 875–882
Bokarewa M et al. (2005) Resistin, an adipokine with potent proinflammatory properties. J Immunol 174: 5789–5795
Distler JH et al. (2005) Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum 52: 3337–3348
Knijff-Dutmer EA et al. (2002) Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum 46: 1498–1503
Berckmans RJ et al. (2002) Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum 46: 2857–2866
Berckmans RJ et al. (2005) Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther 7: R536–R544
Kim SH et al. (2005) Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol 174: 6440–6448
Zhang HG et al. (2006) A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol 176: 7385–7393
McIntyre KW et al. (2003) A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 48: 2652–2659
Sweeney SE and Firestein GS (2004) Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol 16: 231–237
Firestein GS (2006) Inhibiting inflammation in rheumatoid arthritis. N Engl J Med 354: 80–82
Görtz B et al. (2005) Tumour necrosis factor activates the mitogen-activated protein kinases p38α and ERK in the synovial membrane in vivo. Arthritis Res Ther 7: R1140–R1147
Mbalaviele G et al. (2006) Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther 317: 1044–1053
Medicherla S et al. (2006) A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther 318: 132–141
A study of P38 inhibitor (4) in patients with active rheumatoid arthritis (RA) on stable methotrexate therapy [www.clinicaltrials.gov/ct/show/NCT00316771?order=1] (accessed 28 March 2007)
Han Z et al. (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108: 73–81
Chabaud-Riou M and Firestein GS (2004) Expression and activation of mitogen-activated protein kinase kinase-3 and -6 in rheumatoid arthritis. Am J Pathol 164: 177–184
O'Shea JJ et al. (2005) New strategies for immunosuppression: interfering with cytokines by targeting the JAK/STAT pathway. Curr Opin Rheumatol 17: 305–311
Egan PJ et al. (2003) Suppressor of cytokine signaling-1 regulates acute inflammatory arthritis and T cell activation. J Clin Invest 111: 915–924
Wong PK et al. (2006) SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest 116: 1571–1581
Shouda T et al. (2001) Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest 108: 1781–1788
Kremer JM et al. (2006) A randomized, double-blind, placebo-controlled trial of 3 dose levels of CP-690,550 versus placebo in the treatment of active rheumatoid arthritis. In Program and abstracts of the American College of Rheumatology 70th Annual Meeting; November 11–15, 2006: Washington, DC.
Weimar W et al. (2006) Phase 1 evaluation of the safety of a JAK3 inhibitor, CP-690,550 in stable kidney transplant recipients [abstract]. Transplantation 82 (Suppl 2): 86
Comparison of six CP-690,550 doses vs placebo, each combined with methotrexate, for the treatment of rheumatoid arthritis [www.clinicaltrials.gov/ct/show/NCT00413660?order=2] (accessed 28 March 2007)
Ospelt C et al. (2005) Gene analysis for exploring the effects of drugs in rheumatoid arthritis. Arthritis Rheum 52: 2248–2256
Bongartz T et al. (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295: 2275–2285
Setoguchi S et al. (2006) Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum 54: 2757–2764
Acknowledgements
IH Tarner was funded in part by the Deutsche Forschungsgemeinschaft. U Müller-Ladner was funded by the Deutsche Forschungsgemeinschaft. S Gay was funded by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tarner, I., Müller-Ladner, U. & Gay, S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 3, 336–345 (2007). https://doi.org/10.1038/ncprheum0506
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0506
This article is cited by
-
Berberis lycium fruit extract and its phytoconstituents berberine and rutin mitigate collagen–CFA-induced arthritis (CIA) via improving GSK3β/STAT/Akt/MAPKs/NF-κB signaling axis mediated oxi-inflammation and joint articular damage in murine model
Inflammopharmacology (2022)
-
Protective effect of methyl gallate on murine antigen-induced arthritis by inhibiting inflammatory process and bone erosion
Inflammopharmacology (2022)
-
Extracellular DNA and autoimmune diseases
Cellular & Molecular Immunology (2018)
-
Molecular mechanisms regulating NETosis in infection and disease
Seminars in Immunopathology (2013)
-
Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate
Arthritis Research & Therapy (2011)