Abstract
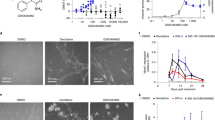
Azacitidine (Vidaza®, Pharmion Corp., Boulder, CO, USA) and decitabine (Dacogen™, SuperGen, Inc., Dublin, CA, USA, and MGI Pharma, Inc., Bloomington, MN, USA) are DNA methyltransferase (DNMT) inhibitors that have clinical activity in patients with myelodysplastic syndromes. Mechanism-based laboratory studies suggest that clinical optimization of therapy with DNMT inhibitors needs to include optimizing intracellular drug uptake and maximizing drug exposure over time while still using lower doses to avoid cytotoxicity. Clinical studies suggest that increased dose intensity and multiple cycles of administration substantially increase response rates. Other strategies for optimizing the efficacy of DNMT inhibitor therapy also include identification of patients that are best qualified for treatment, and defining in vivo mechanisms of patient responses. In the future, combination strategies to increase gene reactivation and to take advantage of increased expression of target genes may be critical for achieving optimal results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Aul C et al. (2002) Myelodysplastic syndromes. Diagnosis and therapeutic strategies [in German]. Med Klin (Munich) 97: 666–676
Kurzrock R (2002) Myelodysplastic syndrome overview. Semin Hematol 39: 18–25
Cooper DN (1983) Eukaryotic DNA methylation. Hum Genet 64: 315–333
Jones PA et al. (1983) DNA modification, differentiation, and transformation. J Exp Zool 228: 287–295
Ando T et al. (2000) Decitabine (5-Aza-2'-deoxycytidine) decreased DNA methylation and expression of MDR-1 gene in K562/ADM cells. Leukemia 14: 1915–1920
Ley TJ et al. (1983) DNA methylation and globin gene expression in patients treated with 5-azacytidine. Prog Clin Biol Res 134: 457–474
Gattei V et al. (1993) In vitro and in vivo effects of 5-aza-2'-deoxycytidine (Decitabine) on clonogenic cells from acute myeloid leukemia patients. Leukemia 7 (Suppl 1): 42–48
Leone G et al. (2002) DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica 87: 1324–1341
Blanchard F et al. (2003) DNA methylation controls the responsiveness of hepatoma cells to leukemia inhibitory factor. Hepatology 38: 1516–1528
Silverman LR et al. (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20: 2429–2440
Saba H et al. (2004) First report of the phase III North American trial of decitabine in advanced myelodysplastic syndrome (MDS) [abstract]. Blood 104: 23A
Kantarjian HM et al. (2004) Decitabine low-dose schedule (100 mg/m(2)/course) in myelodysplastic syndrome (MDS). Comparison of 3 different dose schedules. Blood 104: 402A–403A
Issa J-P et al. (2004) Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 103: 1635–1640
Kaminskas E et al. (2005) Approval summary azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res 11: 3604–3608
Anon (2003) Decitabine: 2'-deoxy-5-azacytidine, Aza dC, DAC, dezocitidine, NSC 127716. Drugs R&D 4: 352–358
Saba H et al. Advanced myelodysplastic syndromes: patient subset results of a phase III trial comparing decitabine with supportive care [abstract]. J Clin Oncol 23: 6543
Saiki JH et al. (1978) 5-Azacytidine in acute leukemia. Cancer 42: 2111–2114
Vogler WR et al. (1976) 5-Azacytidine (NSC 102816): a new drug for the treatment of myeloblastic leukemia. Blood 48: 331–337
Von Hoff DD et al. (1976) 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann Intern Med 85: 237–245
Schiffer CA et al. (1982) Treatment of the blast crisis of chronic myelogenous leukemia with 5-azacitidine and VP-16–213. Cancer Treat Rep 66: 267–271
Lee EJ et al. (1990) Low dose 5-azacytidine is ineffective for remission induction in patients with acute myeloid leukemia. Leukemia 4: 835–838
Kornblith AB et al. (2002) Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a Cancer and Leukemia Group B study. J Clin Oncol 20: 2441–2452
Schwartsmann G et al. (1997) Decitabine (5-Aza-2'-deoxycytidine; DAC) plus daunorubicin as a first line treatment in patients with acute myeloid leukemia: preliminary observations. Leukemia 11 (Suppl 1): 28–31
Petti MC et al. (1993) Pilot study of 5-aza-2'-deoxycytidine (Decitabine) in the treatment of poor prognosis acute myelogenous leukemia patients: preliminary results. Leukemia 7 (Suppl 1): 36–41
Willemze R et al. (1997) A randomized phase II study on the efects of 5-Aza-2'-deoxycytidine combined with either amsacrine or idarubicin in patients with relapsed acute leukemia: an EORTC Leukemia Cooperative Group phase II study (06893). Leukemia 11 (Suppl 1): 24–27
Wijermans PW et al. (1997) Continuous infusion of low-dose 5-Aza-2'-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia 11 (Suppl 1): 19–23
Wijermans PW et al. (2000) Low-dose 5-aza-2'-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol 18: 956–962
Saba HI and Wijermans PW (2005) Decitabine in myelodysplastic syndromes. Semin Hematol 42 (Suppl 2): S23–S31
Miller KB (2000) Myelodysplastic syndromes. Curr Treat Options Oncol 1: 63–69
Candoni A et al. (2004) Targeted therapies in myelodysplastic syndromes: ASH 2003 review. Semin Hematol 41: 13–20
Mufti G et al. (2003) Myelodysplastic syndrome. Hematology (Am Soc Hematol Educ Program): 176–199
Alessandrino EP et al. (2002) Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica 87: 1286–1306
Cutler CS et al. (2004) A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104: 579–585
List A et al. (2005) Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352: 549–557
Lee EJ et al. (1990) Low dose 5-azacytidine is ineffective for remission induction in patients with acute myeloid leukemia. Leukemia 4: 835–838
Petti MC et al. (1993) Pilot study of 5-aza-2'-deoxycytidine (Decitabine) in the treatment of poor prognosis acute myelogenous leukemia patients: preliminary results. Leukemia 7 (Suppl 1): 36–41
Issa J-P et al. (2005) Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol 23: 3948–3956
van den Bosch J et al. (2004) The effects of 5-aza-2'-deoxycytidine (Decitabine) on the platelet count in patients with intermediate and high-risk myelodysplastic syndromes. Leuk Res 28: 785–790
Issa J-P (2003) Decitabine. Curr Opin Oncol 15: 446–451
Jones PA and Taylor SM (1980) Cellular differentiation, cytidine analogs and DNA methylation. Cell 20: 85–93
Momparler RL (2005) Pharmacology of 5-Aza-2'-deoxycytidine (decitabine). Semin Hematol 42: S9–S16
Issa J-P et al. (2005) Azacitidine. Nat Rev Drug Discov 4: 275–276
Maio M et al. (2003) Epigenetic targets for immune intervention in human malignancies. Oncogene 22: 6484–6488
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Prof Jean-Pierre Issa has received consulting fees, lecture fees, and research support from SuperGen Corp., MGI Pharma Inc., and Pharmion Corp.
Rights and permissions
About this article
Cite this article
Issa, JP. Optimizing therapy with methylation inhibitors in myelodysplastic syndromes: dose, duration, and patient selection. Nat Rev Clin Oncol 2 (Suppl 1), S24–S29 (2005). https://doi.org/10.1038/ncponc0355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0355
This article is cited by
-
Comparison of clinical outcomes between peripheral blood stem cells and peripheral blood stem cells plus bone marrow in myelodysplastic syndrome patients with haploidentical transplantation
Bone Marrow Transplantation (2023)
-
Human umbilical cord mesenchymal stem cells in type 2 diabetes mellitus: the emerging therapeutic approach
Cell and Tissue Research (2021)
-
Inhibition of DNMT1 and ERRα crosstalk suppresses breast cancer via derepression of IRF4
Oncogene (2020)
-
Decitabine assists umbilical cord-derived mesenchymal stem cells in improving glucose homeostasis by modulating macrophage polarization in type 2 diabetic mice
Stem Cell Research & Therapy (2019)
-
Overview of DNA methylation in adult diffuse gliomas
Brain Tumor Pathology (2019)