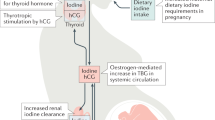
Abstract
The diagnosis of Graves' disease in pregnancy can be complex because of normal gravid physiologic changes in thyroid hormone metabolism. Mothers with active Graves' disease should be treated with antithyroid drugs, which impact both maternal and fetal thyroid function. Optimally, the lowest possible dose should be used to maintain maternal free thyroxine levels at or just above the upper limit of the normal nonpregnant reference range. Fetal thyroid function depends on the balance between the transplacental passage of thyroid-stimulating maternal antibodies and thyroid-inhibiting antithyroid drugs. Elevated levels of serum maternal anti-TSH-receptor antibodies early in the third trimester are a risk factor for fetal hyperthyroidism and should prompt evaluation of the fetal thyroid by ultrasound, even in women with previously ablated Graves' disease. Maternal antithyroid medication can be modulated to treat fetal hyperthyroidism. Serum TSH and either total or free thyroxine levels should be measured in fetal cord blood at delivery in women with active Graves' disease, and those with a history of 131I-mediated thyroid ablation or thyroidectomy who have anti-TSH-receptor antibodies. Neonatal thyrotoxicosis can occur in the first few days of life after clearance of maternal antithyroid drug, and can last for several months, until maternal antibodies are also cleared.
Key Points
-
First-line therapy for Graves' disease during pregnancy includes antithyroid drugs (preferably propylthiouracil)
-
Prescribed doses should be as low as possible to maintain maternal serum free T4 levels at or just above the upper limit of the normal nonpregnant range, or total T4 levels at 1.5-times the normal nonpregnant reference range
-
If continued administration of antithyroid medication is not possible, second-trimester thyroidectomy can be considered; patients should receive β-adrenergic blockade and iodide therapy preoperatively
-
All women with active Graves' disease, and levothyroxine-replaced patients with a history of 131I-mediated ablation or thyroidectomy, should have their anti-TSH-receptor antibody (TRAb) levels measured at 26–28 weeks gestation to evaluate the risk for fetal hyperthyroidism
-
To assess fetal thyroid function, fetal ultrasound at 28–32 weeks should be performed if there is evidence of active maternal Graves' disease (elevated maternal TRAb levels, or maternal requirement for antithyroid medication)
-
Serum TSH and total T4 or free T4 concentrations should be measured in fetal cord blood at delivery in women with active Graves' disease or positive TRAb screen after 131I-mediated ablation or thyroidectomy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mestman JH (1997) Hyperthyroidism in pregnancy. Clin Obstet Gynecol 40: 45–64
Burrow GN (1993) Thyroid function and hyperfunction during gestation. Endocr Rev 14: 194–202
Casey BM et al. (2006) Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 107: 337–341
Davis LE et al. (1989) Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol 160: 63–70
Millar LK et al. (1994) Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol 84: 946–949
Anselmo J et al. (2004) Fetal loss associated with excess thyroid hormone exposure. JAMA 292: 691–695
Momotani N et al. (1984) Maternal hyperthyroidism and congenital malformation in the offspring. Clin Endocrinol (Oxf) 20: 695–700
Phoojaroenchanachai M et al. (2001) Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin Endocrinol (Oxf) 54: 365–370
Mitsuda N et al. (1992) Risk factors for developmental disorders in infants born to women with Graves disease. Obstet Gynecol 80: 359–364
Burrow GN (1985) The management of thyrotoxicosis in pregnancy. N Engl J Med 313: 562–565
Demers LM and Spencer CA (2003) Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf) 58: 138–140
Sapin R et al. (2004) Free thyroxine measured with equilibrium dialysis and nine immunoassays decreases in late pregnancy. Clin Lab 50: 581–584
Spencer CA et al. (2005) Thyroid reference ranges in pregnancy: studies of an iodine-sufficient pregnant cohort [abstract #O 43]. Thyroid 15 (Suppl 1): S-16
Panesar NS et al. (2001) Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 38: 329–332
Casey BM et al. (2005) Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105: 239–245
Haddow JE et al. (2004) The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen 11: 170–174
Glinoer D et al. (1993) Serum levels of intact human chorionic gonadotropin (HCG) and its free α and β subunits, in relation to maternal thyroid stimulation during normal pregnancy. J Endocrinol Invest 16: 881–888
Goodwin TM et al. (1992) Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am J Obstet Gynecol 167: 648–652
Amino N et al. (1982) Aggravation of thyrotoxicosis in early pregnancy and after delivery in Graves' disease. J Clin Endocrinol Metab 55: 108–112
Marqusee E et al. (1997) Thyroiditis after pregnancy loss. J Clin Endocrinol Metab 82: 2455–2457
Tan JY et al. (2002) Transient hyperthyroidism of hyperemesis gravidarum. BJOG 109: 683–688
Luton D et al. (2005) Management of Graves' disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab 90: 6093–6098
Amino N et al. (2003) No increase of blocking type anti-thyrotropin receptor antibodies during pregnancy in patients with Graves' disease. J Clin Endocrinol Metab 88: 5871–5874
Zakarija M and McKenzie JM (1983) Pregnancy-associated changes in the thyroid-stimulating antibody of Graves' disease and the relationship to neonatal hyperthyroidism. J Clin Endocrinol Metab 57: 1036–1040
Wing DA et al. (1994) A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am J Obstet Gynecol 170: 90–95
Marchant B et al. (1977) The placental transfer of propylthiouracil, methimazole and carbimazole. J Clin Endocrinol Metab 45: 1187–1193
Mortimer RH et al. (1997) Methimazole and propylthiouracil equally cross the perfused human term placental lobule. J Clin Endocrinol Metab 82: 3099–3102
Gardner DF et al. (1986) Pharmacology of propylthiouracil (PTU) in pregnant hyperthyroid women: correlation of maternal PTU concentrations with cord serum thyroid function tests. J Clin Endocrinol Metab 62: 217–220
Momotani N et al. (1997) Effects of propylthiouracil and methimazole on fetal thyroid status in mothers with Graves' hyperthyroidism. J Clin Endocrinol Metab 82: 3633–3636
Momotani N et al. (1986) Antithyroid drug therapy for Graves' disease during pregnancy. Optimal regimen for fetal thyroid status. N Engl J Med 315: 24–28
Cheron RG et al. (1981) Neonatal thyroid function after propylthiouracil therapy for maternal Graves' disease. N Engl J Med 304: 525–528
Momotani N et al. (2006) Anti-thyroid drug therapy for Graves' disease during pregnancy: mildest thyrotoxic maternal free thyroxine concentrations to avoid fetal hypothyroidism [abstract #87]. Presented at the 77th Annual Meeting of the American Thyroid Association: 2006 October 11–15, Phoenix, AZ
Wolf D et al. (2006) Antenatal carbimazole and choanal atresia: a new embryopathy. Arch Otolaryngol Head Neck Surg 132: 1009–1011
Di Gianantonio E et al. (2001) Adverse effects of prenatal methimazole exposure. Teratology 64: 262–266
Clementi M et al. (1999) Methimazole embryopathy: delineation of the phenotype. Am J Med Genet 83: 43–46
Van Dijke CP et al. (1987) Methimazole, carbimazole, and congenital skin defects. Ann Intern Med 106: 60–61
Mujtaba Q and Burrow GN (1975) Treatment of hyperthyroidism in pregnancy with propylthiouracil and methimazole. Obstet Gynecol 46: 282–286
Hayashida CY et al. (1990) Neonatal hepatitis and lymphocyte sensitization by placental transfer of propylthiouracil. J Endocrinol Invest 13: 937–941
Eisenstein Z et al. (1992) Intellectual capacity of subjects exposed to methimazole or propylthiouracil in utero. Eur J Pediatr 151: 558–559
Messer PM et al. (1990) Antithyroid drug treatment of Graves' disease in pregnancy: long-term effects on somatic growth, intellectual development and thyroid function of the offspring. Acta Endocrinol (Copenh) 123: 311–316
Redmond GP (1982) Propranolol and fetal growth retardation. Semin Perinatol 6: 142–147
Momotani N et al. (1992) Effects of iodine on thyroid status of fetus versus mother in treatment of Graves' disease complicated by pregnancy. J Clin Endocrinol Metab 75: 738–744
Stoffer SS and Hamburger JI (1976) Inadvertent 131I therapy for hyperthyroidism in the first trimester of pregnancy. J Nucl Med 17: 146–149
Brosco JP et al. (2006) Impact of specific medical interventions on reducing the prevalence of mental retardation. Arch Pediatr Adolesc Med 160: 302–309
Rose SR et al. (2006) Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 117: 2290–2303
Brodsky JB et al. (1980) Surgery during pregnancy and fetal outcome. Am J Obstet Gynecol 138: 1165–1167
Fisher DA (1997) Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol 40: 16–31
Szinnai G et al. (2007) Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus. J Clin Endocrinol Metab 92: 70–76
Chopra IJ (1992) Fetal and neonatal hyperthyroidism. Thyroid 2: 161–163
Di Cosmo C et al. (2006) The sodium-iodide symporter expression in placental tissue at different gestational age: an immunohistochemical study. Clin Endocrinol (Oxf) 65: 544–548
Bidart JM et al. (2000) Expression of Na+/I– symporter and Pendred syndrome genes in trophoblast cells. J Clin Endocrinol Metab 85: 4367–4372
Mitchell AM et al. (2001) Sodium iodide symporter (NIS) gene expression in human placenta. Placenta 22: 256–258
Vulsma T et al. (1989) Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 321: 13–16
de Escobar GM et al. (2004) Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18: 225–248
Gitlin D (1971) Development and metabolism of the immune globulins. In Immunologic Incompetence 3–16, (Eds Kagan BM and Stiehm ER) Chicago: Year Book Medical Publishers, Inc.
Nachum Z et al. (2003) Graves' disease in pregnancy: prospective evaluation of a selective invasive treatment protocol. Am J Obstet Gynecol 189: 159–165
Polak M et al. (2004) Fetal and neonatal thyroid function in relation to maternal Graves' disease. Best Pract Res Clin Endocrinol Metab 18: 289–302
Wallace C et al. (1995) Fetal thyrotoxicosis: a case report and recommendations for prediction, diagnosis, and treatment. Thyroid 5: 125–128
Polak M et al. (2006) Congenital hyperthyroidism: the fetus as a patient. Horm Res 65: 235–242
Cohen O et al. (2003) Serial in utero ultrasonographic measurements of the fetal thyroid: a new complementary tool in the management of maternal hyperthyroidism in pregnancy. Prenat Diagn 23: 740–742
Van Loon AJ et al. (1995) In utero diagnosis and treatment of fetal goitrous hypothyroidism, caused by maternal use of propylthiouracil. Prenat Diagn 15: 599–604
Ochoa-Maya MR et al. (1999) Resolution of fetal goiter after discontinuation of propylthiouracil in a pregnant woman with Graves' hyperthyroidism. Thyroid 9: 1111–1114
LeBeau SO and Mandel SJ (2006) Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am 35: 117–136
McNab T and Ginsberg J (2005) Use of anti-thyroid drugs in euthyroid pregnant women with previous Graves' disease. Clin Invest Med 28: 127–131
Bruinse HW et al. (1988) Fetal treatment for thyrotoxicosis in non-thyrotoxic pregnant women. Fetal Ther 3: 152–157
Serup J and Petersen S (1977) Hyperthyroidism during pregnancy treated with propylthiouracil. The significance of maternal and foetal parameters. Acta Obstet Gynecol Scand 56: 463–466
Davidson KM et al. (1991) Successful in utero treatment of fetal goiter and hypothyroidism. N Engl J Med 324: 543–546
Kilpatrick S (2003) Umbilical blood sampling in women with thyroid disease in pregnancy: is it necessary? Am J Obstet Gynecol 189: 1–2
Skuza KA et al. (1996) Prediction of neonatal hyperthyroidism in infants born to mothers with Graves disease. J Pediatr 128: 264–268
Matsuura N et al. (1988) TSH-receptor antibodies in mothers with Graves' disease and outcome in their offspring. Lancet 1: 14–17
Peleg D et al. (2002) The relationship between maternal serum thyroid-stimulating immunoglobulin and fetal and neonatal thyrotoxicosis. Obstet Gynecol 99: 1040–1043
Kempers MJ et al. (2003) Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab 88: 5851–5857
Acknowledgements
GW Chan is supported by NIH grant 2-T32-DK007314-26. We thank C Spencer for supplying the data used for Figure 1. Désirée Lie, University of California, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chan, G., Mandel, S. Therapy Insight: management of Graves' disease during pregnancy. Nat Rev Endocrinol 3, 470–478 (2007). https://doi.org/10.1038/ncpendmet0508
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0508
This article is cited by
-
Unusual onset of thyroid associated orbitopathy during pregnancy: case report and review of literature
BMC Endocrine Disorders (2020)
-
Embolic suppurative thyroiditis with concurrent carcinoma in pregnancy: lessons in management through a case report
Thyroid Research (2015)
-
Thyroid diseases during pregnancy: A number of important issues
Hormones (2015)