Abstract
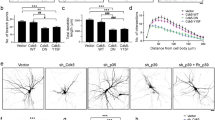
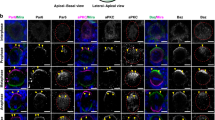
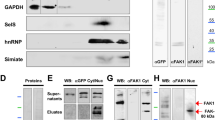
PAR-3 (partitioning-defective gene 3) is essential for cell polarization in many contexts, including axon specification. However, polarity proteins have not been implicated in later steps of neuronal differentiation, such as dendritic spine morphogenesis. Here, we show that PAR-3 is necessary for normal spine development in primary hippocampal neurons. Depletion of PAR-3 causes the formation of multiple filopodia- and lamellipodia-like dendritic protrusions — a phenotype similar to neurons expressing activated Rac. PAR-3 regulates spine formation by binding the Rac guanine nucleotide-exchange factor (GEF) TIAM1, and spatially restricting it to dendritic spines. Thus, a balance of PAR-3 and TIAM1 is essential to modulate Rac–GTP levels and to allow spine morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hering, H. & Sheng, M. Dendritic spines: structure, dynamics and regulation. Nature Rev. Neurosci. 2, 880–888 (2001).
Goda, Y. & Davis, G. W. Mechanisms of synapse assembly and disassembly. Neuron 40, 243–264 (2003).
Fiala, J. C., Spacek, J. & Harris, K. M. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res. Brain Res. Rev. 39, 29–54 (2002).
Halpain, S., Spencer, K. & Graber, S. Dynamics and pathology of dendritic spines. Prog. Brain Res. 147, 29–37 (2005).
Macara, I. G. Parsing the polarity code. Nature Rev. Mol. Cell Biol. 5, 220–231 (2004).
Ohno, S. Intercellular junctions and cellular polarity: the PAR–αPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13, 641–648 (2001).
Kemphues, K. J., Priess, J. R., Morton, D. G. & Cheng, N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311–320 (1988).
Schneider, S. Q. & Bowerman, B. Cell polarity and the cytoskeleton in the Caenorhabditis elegans zygote. Annu. Rev. Genet. 37, 221–249 (2003).
Doe, C. Q. & Bowerman, B. Asymmetric cell division: fly neuroblast meets worm zygote. Curr. Opin. Cell Biol. 13, 68–75. (2001).
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Shi, S. H., Jan, L. Y. & Jan, Y. N. Hippocampal neuronal polarity specified by spatially localized mPar3–mPar6 and PI 3-kinase activity. Cell 112, 63–75 (2003).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Wei, X. et al. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev. Biol. 269, 286–301 (2004).
Chen, X. & Macara, I. G. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nature Cell Biol. 7, 262–269 (2005).
Nakayama, A. Y., Harms, M. B. & Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 (2000).
Nishimura, T. et al. PAR-6–PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF–Tiam1. Nature Cell Biol. 7, 270–277 (2005).
Kunda, P. et al. Evidence for the involvement of Tiam1 in axon formation. J. Neurosci. 21, 2361–2372 (2001).
Tanaka, M. et al. Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J. 23, 1075–1088 (2004).
Tolias, K. F. et al. The Rac1 GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 (2005).
Stam, J. C. et al. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. 272, 28447–28454 (1997).
Sala, C. et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 (2001).
Fischer, M. et al. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nature Neurosci. 3, 887–894 (2000).
Mertens, A. E. et al. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J. Cell Biol. 170, 1029–1037 (2005).
Hirose, T. et al. Involvement of ASIP–PAR-3 in the promotion of epithelial tight junction formation. J. Cell Sci. 115, 2485–2495 (2002).
Lin, D. et al. A mammalian PAR-3–PAR-6 complex implicated in Cdc42–Rac1 and αPKC signalling and cell polarity. Nature Cell Biol. 2, 540–547 (2000).
Goslin, K., Asmussen, H. & Banker, G. Rat Hippocampal Neurons in Low-Density Culture (ed. Goslin, G. B.) (MIT Press, Cambridge, MA, 1998).
Zhang, H., Webb, D. J., Asmussen, H. & Horwitz, A. F. Synapse formation is regulated by the signalling adaptor GIT1. J. Cell Biol. 161, 131–142 (2003).
Acknowledgements
We thank J. Fawcett and T. Pawson for providing the PAR-3 antibody, X. Chen and L. Gao for constructs and helpful discussions, and Y. Qin for help with culture of the hippocampal neurons. We would also like to extend our gratitude to A. Spang (Friedrich-Miescher-Laboratorium, Tübingen, Germany) and D. Webb (Vanderbilt University, Nashville, TN) for comments on the manuscript. This work was supported by grant GM070902 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1, S2, S3 and S4 (PDF 819 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Macara, I. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol 8, 227–237 (2006). https://doi.org/10.1038/ncb1368
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1368
This article is cited by
-
Impact of Copper Nanoparticles and Copper Ions on Transcripts Involved in Neural Repair Mechanisms in Rainbow Trout Olfactory Mucosa
Archives of Environmental Contamination and Toxicology (2023)
-
Genome-wide translation control analysis of developing human neurons
Molecular Brain (2022)
-
Genome-wide association and genotype by environment interactions for growth traits in U.S. Red Angus cattle
BMC Genomics (2022)
-
The polarity protein PARD3 and cancer
Oncogene (2021)
-
MiR-190a potentially ameliorates postoperative cognitive dysfunction by regulating Tiam1
BMC Genomics (2019)