Abstract
We report a case of hemangiopericytoma of the soft palate of 60-year-old patient, who noticed a mass of the soft palate and experienced difficulty in speaking. We found a pediculate, hard, elastic mass measuring 38 mm (cross-sectional diameter). Computed tomography (CT) scans and dynamic magnetic resonance imaging (MRI) confirmed irregularly shaped mass and revealed a heterogeneous internal composition, consistent with vascular tumors. We excised the tumor under general anesthesia. Histopathological diagnosis was based on positive immunoreactivity of CD99 and vimentin and weak, positive staining of CD34. Three and half years following tumor excision, there is no recurrence or metastasis.
Similar content being viewed by others
Introduction
Hemangiopericytomas are soft tissue sarcomas that originate in pericytes, cells of mesenchymal origin that partially surround the endothelial cells of capillaries and small veins where they assist in the regulation of blood flow. Hemangiopericytomas surround irregularly formed vascular tissue. As sarcomas, hemangiopericytomas can occur in bone and soft tissue, including muscle, the liver, and the heart. Fortunately, hemangiopericytomas are rare and typically benign.1,2
In the United States, 6 000–7 000 soft-tissue sarcomas are reported each year, but of these, less than 1% are diagnosed as hemangiopericytomas. Similarly, less than 1% of the 2 000 bone sarcomas reported each year are hemangiopericytomas. They are most commonly diagnosed in patients between the ages of 30 and 50 years, but they have also been documented in children. When diagnosed, it is often as low-grade benign sarcomas, and they commonly present as slow-growing, and painless tumors of the extremities, primarily the femur and proximal tibia. However, malignant hemangiopericytomas with more aggressive phenotypes have also been documented.1,2
As sarcomas, hemangiopericytomas are graded based on histologic and biologic parameters. However, because the pericytes, in which they originate, possess characteristics of both smooth muscle and endothelial cells, differentiating these from other cell types is often challenging. Accordingly, the diagnosis of hemangiopericytoma is made on the basis of distinct architectural patterns exhibited histologically.3
Case report
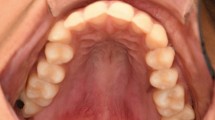
A 60-year-old male was referred to and examined at Clinic of Maxillofacial Surgery in Tokyo Medical and Dental University Dental Hospital in January 2006, after reporting the sensation of foreign body in his mouth and experiencing difficulty speaking. He was of average height and weight; his family medical history was unremarkable, but he had been receiving drug treatment for non-insulin dependent diabetes since age 50. The patient noticed a foreign body sensation in the left soft palate in around 2000 but did not seek treatment, as it was not painful. In December 2004, he experienced respiratory discomfort and was examined in the ear-nose-thorat (ENT) department of a university hospital. A diagnosis was not reached, despite the use of computed tomography (CT), magnetic resonance imaging (MRI) and aspiration cytology, and the patient stopped seeking medical consultation at that hospital. In December 2005, he was examined at a local dental clinic, after experiencing dysphagia, and was referred to our hospital. Upon initial examination, we discovered an elastic, hard, everted, pedunculated tumor 38 mm in maximum diameter with a 15-mm in the left soft palate (Figure 1a).
Intraoral images at the first visit. (a) Photograph of the pedunculate hemangiopericytoma in the soft palate, taken during the initial examination. (b) Gadolinium-enhanced T1-weighted MRI image showing the irregularly-shaped, protruding mass, measuring 45 mm×20 mm, in the left soft palate of the patient. (c) Contrast CT scan image showing the internally heterogeneous composition and irregular borders of the tumor. CT, computed tomography; MRI, magnetic resonance imaging.
MRI confirmed the presence of an irregularly shaped, protruded tumor, 45 mm×20 mm in size, in the left soft palate of the patient. The appearance of the tumor was similar to muscle on T1-weighted images, and displayed heterogeneous hyperintensity of its internal structure. Upon gadolinium contrast enhancement, on T2-weighted images, the internal structure displayed both faintly intensified and strongly intensified areas. Dynamic MRI also revealed the heterogeneous composition, with some areas exhibiting a rapid increase followed by a gradual decrease and other regions exhibiting a rapid increase followed by a plateau (Figure 1b).
CT images of the patient confirmed the findings of MRI, revealing an internally heterogeneous tumor with irregular borders of size 38 mm×30 mm, evident mainly in the left soft palate. CT scans also indicated that there was no tumor-induced maxillary destruction (Figure 1c).
Biopsy of the tumor was performed in January 2006, and hemangiopericytoma was diagnosed. The biopsy specimen had a high cell density and as many as 17 mitotic figures/high-power field (HPF), indicating the possibility of malignancy.
Resection was scheduled, and preoperative speech tests were conducted in February 2006. Articulation tests revealed lateral articulation, rhinolalia clausa (hyponasality), distortion of velar consonants (consonant that is pronounced with the back part of the tongue against the soft palate or velum), resulting from contact between the back of the tongue and the tumor. Alveolar fricatives (consonants produced by forcing air through a narrow channel made by placing two articulators close together) and affricates (consonants that begin as tops but release as fricatives) were also affected, due to partial obstruction of expiration by the tumor.
In February 2006, the patient was admitted to Tokyo Medical and Dental University Dental Hospital and the tumor was resected under general anesthesia. A 5-mm safety margin was left at the base of the tumor, resection was performed by using an electric scalpel, and the mucosal defect was covered with a graft of artificial dermis. The patient’s course was uneventful and he was discharged in March 2006. Since that time he has remained under observation. In speech and articulation tests, performed two months postoperatively, rhinolalia clausa and obscuration were no longer evident. At 3.5 years after surgery, there was no evidence of recurrence or metastasis, and the pronunciation defect had disappeared.
Macroscopically, a cross-section of the resected tumor with 5-mm surgical margin was pale, yellowish-brown in color, and solid, with lobulated nodules (data not shown). Microscopically, a tangled arrangement of fusiform and solid tumor cells were observed with numerous thin-walled vessels (Figure 2a), some of which were branched in ‘stag-horn’ shapes (arrows in Figure 2b). Silver staining showed a network of reticular fibers between tumor cells (data not shown). Tumor cells revealed a high mitotic index score (21 mitotic figures/10 HPFs), and sometimes showed a cytological atypia (bizarre, hyperchromatic nuclei). Immunohistochemical staining showed that tumor cells were focally weakly positive for CD34 (Figure 2c), and strongly diffuse positive for vimentin and CD99 (Figure 2d), negative for S-100, EMA and Bcl-2 (data not shown).
Histological findings. (a) Lower magnification shows the dense, cellular rich tumor cells arranged around the numerous thin-walled vessels (HE stain, original magnification ×100). (b) Within tumor nests, thin-walled branching vessels, stag-horn configuration are observed (arrowed, HE stain, original magnification ×400). (c) Immunohistochemical staining shows tumor cells with focally weak positivity for CD34 (original magnification ×400). (d) Immunohistochemical staining shows tumor cells with diffused strong positivity for CD99 (original magnification ×400). HE, hematoxylin and eosin.
Discussion
Hemangiopericytoma was defined in 1942 by Stout and Murray4 as a tumor that arises from pericytes surrounding capillary vessels. It can arise anywhere capillary vessels are found,5 but is most commonly seen in the limbs and retroperitoneum.6,7,8,9 Some reports have indicated that it also tends to arise in the head and neck,10,11 but its occurrence in the mouth is regarded as rare. In the 2002 World Heath Organization (WHO) classification,12 it is not categorized as neither benign nor malignant, but is regarded as a tumor with potential low malignancy. As far as we have been able to determine from a search of the literature, including the present case, 16 cases of hemangiopericytoma of the mouth have been reported worldwide,10,11,12,13,14,15,16,17,18,19 of which eight were described as malignant (Table 1).
In general, hemangiopericytoma is believed to occur at an equal rate in both sexes and all age groups.5,6 Our count of its occurrence in the mouth found that the age distribution ranged from 13 to 91 years, with a median age of 44.5 years, and a slightly elevated incidence among women (male:female=1:1.3). The most common site of origin was the palate (five cases), followed by the mandible and the lower lip. Clinical symptoms most commonly comprised a mass or swelling, and associated pain was not reported in any of the case. Instead, most patients simply complain about the presence of the tumor. Tumor sizes ranged from 3 mm to 60 mm, with a median size of 23 mm. In the present case, the tumor was located at the back of the palate and was larger than this average (38 mm). In the present case, the size and location of the tumor led to subjective symptoms, including difficulties speaking and swallowing. Speech tests demonstrated an improvement in articulation following surgery.
Regarding the physical characteristic of hemangiopericytomas, they were elastic-hard (reported in four cases, including this case); Adhesions to the surrounding tissue were reported in one case and no adhesions in two (including the present case). Although the present case included a protruding lesion, most of the oral hemangiopericytomas in the literature were invasive, and only one other pedunculated tumor like the one presented here has been reported.
Few reports of imaging results exist in the literature. CT scans are described for three of the cases.12,14,18,19 CT depicted ‘roundish’, localized tumors in most cases. When contrast agent was used during CT, the interiors of the tumors were heterogeneously intensified, as in the present case.
MRI revealed internally heterogeneous tumors that were hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging. Again, when contrast agent was used, the interior of the tumor was also heterogeneously intensified by the contrast agent on T1-weighted imaging. Dynamic MRI showed the tumor to comprise a mixture of regions exhibiting a rapid increase and gradual decrease and regions exhibiting a rapid increase followed by a plateau. These CT and MRI results are not diagnostic features of hemangiopericytoma, but are characteristic of vascular tumors.
In terms of treatment, surgical resection was performed in nine cases (including the present case), enucleation in three and resection plus radiotherapy in two. As the tumor in the present case was pedunculated and had extremely well demarcated borders, it was resected with a safety margin of around 5 mm, and there has been no subsequent recurrence. None of the other reports described the safety margin during resection, but some mentioned adhesions to the surrounding tissue,12,14 and in light of the fact that this tumor is believed to be of potential low malignancy and there have been cases of recurrence and metastasis, a safety margin of around 10 mm at least is necessary unless the tumor borders are extremely well demarcated.
Histopathologically, the so-called ‘stag-horn’ sign, formed by proliferation of fusiform to roundish undifferentiated tumor cells in dendritic branches around the capillary vessels, was formerly regarded10 as useful in the diagnosis of hemangiopericytoma. However, because this finding is also present in many other soft-tissue tumors, it is no longer considered a distinguishing characteristic of hemangiopericytoma. In order to distinguished hemangiopericytoma from other solitary fibrous tumors, diagnosis must be based on results of immunostaining. In the present case, the possibility of solitary fibrous tumor was considered because tumor cells were positive for both CD99 and vimentin, but this was ruled out by the weak, positive staining for CD34 (a cluster of differentiation molecule expressed in endothelial cells of blood vessels). Although the 2002 WHO classification does not include clear diagnostic criteria for the grades of malignancy,9 characteristic of malignant hemangiopericytomas are described in the literature. These include increased cellular density and hemorrhage,6,20 necrosis,6,19,20 cellular atypia,20 nuclear polymorphism19 and elevated mitotic figures.6,19,20 Enzinger and Smith6 and Orlando et al.19 reported that mitotic figures of 4 and ≥4/10 HPF, respectively, were associated with malignant lesions. Meanwhile, Batsakis et al.20 found that mitotic figures ≥1/10 HPF and ≥1/20 HPF, in addition to mild or moderate cellular atypia, respectively, were consistent with malignant tumors. Considering the characteristics of the present case—the numerous cellular components (cellular atypia), solid nature of the mass (increased cellular density) and elevated mitotic figures (as high as 17/HPF)—it is likely that the hemangiopericytoma was at least a low-grade malignancy.
In terms of the course and outcome of hemangiopericytoma, Backwinkel et al.5 stated that recurrence or metastasis occurred in 118 of 224 patients (52.2%) with this tumor anywhere in the body, Batsakis et al.20 reported that metastasis occurred in 31 out of 60 patients (51.7%), and O’Brien and Brasfield7 observed it in 13 of 23 patients (56.5%). Our search of the literature for the oral region found that average follow-up period was 45 months, and that local recurrence or distant metastasis occurred in 4 of 16 patients (25.0%), a lower rate than that has been observed in other regions. Although outcome will probably be influenced by factors including site of origin, histopathological malignancy, and treatment method, it has also been reported that hemangiopericytomas of the head and neck tend to be comparatively less malignant.20 Distal metastasis occurred in only two of six patients in whom malignancy was diagnosed. Our patient did not display any signs of recurrence or metastasis 3.5 years following surgical resection. However, recurrence and metastasis peaks at ≥5 years, thus, we will continue to monitor the patient in the present case.
References
Enzinger FM, Weiss SW . Soft Tissue Tumors. 2nd ed. St Louis: Mosby, 1988: 596–613.
Unni KK, Ivins JC, Beabout JW et al. Hemangioma, hemangiopericytoma, and hemangioendothelioma (angiosarcoma) of bone. Cancer 1971; 27( 6): 1403–1414.
Rodríguez-Gil Y, González MA, Carcavilla CB et al. Lines of cell differentiation in solitary fibrous tumor: an ultrastructural and immunohistochemical study of 10 cases. Ultrastruct Pathol 2009; 33( 6): 274–285.
Stout AP, Murray MR . Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericyte. Ann Surg 1942; 116( 1): 26–33.
Backwinkel KD, Diddams JA . Hemangiopericytoma. Report of a case and comprehensive review of the literature. Cancer 1970; 25( 4): 896–901.
Enzinger FM, Smith BH . Hemangiopericytoma. An analysis of 106 cases. Hum Pathol 1976; 7( 1): 61–82.
O'brien P, Brasfield RD . Hemangiopericytoma. Cancer 1965; 18: 249–252.
Hogle WP . Malignant hemangiopericytoma: a clinical overview and case study. Clin J Oncol Nurs 2003; 7( 1): 57–62.
Fletcher CD, Unni K, Mertens F . World health organization. Classification of tumours, pathology and genetics, tumours of soft tissue and bone. Lyon: IARC Press, 2002: 432.
Stout AP . Hemangiopericytoma: a study of twenty five new cases. Cancer 1949; 2( 6): 1027–1035.
Morita N, Sakamoto T, Yamamoto M et al. Hemangiopericytoma of the oral cavity. J Oral Maxillofac Surg 1982; 40( 5): 305–307.
Goldwasser MS, Daw JL . Hemangiopericytoma of the palate: case report. J Oral Maxillofac Surg 1990; 48( 2): 211–215.
Kothari PS, Murphy M, Howells GL et al. Hemangiopericytoma: a report of two cases arising on the lip. Br J Oral Maxillofac Surg 1996; 34( 5): 454–456.
Terakado M, Uehara T, Takigawa T et al. A case of hemangiopericytoma of the buccal mucosa. J Nihon Univ Sch Dent 1997; 39( 4): 211–215.
Carew JF, Singh B, Kraus DH . Hemangiopericytoma of the head and neck. Laryngoscope 1999; 109( 9): 1409–1411.
Billings KR, Fu YS, Calcaterra TC et al. Hemangiopericytoma of the head and neck. Am J Otolaryngol 2000; 21: 238–243.
Moriya S, Tei K, Notani K et al. Malignant hemangiopericytoma of the head and neck: a report of 3 cases. J Oral Maxillofac Surg 2001; 59( 3): 340–345.
Hiraumi H, Kitajiri S, Hirose T, et al. Radiosensitive hemangiopericytoma in the soft palate. Auris Nases Larynx 2002; 29( 1): 95–97.
Orlando GJ, Gil MM, Olivier R . Giant hemangiopericytoma of mandible: a propos of a case: a variant of the surgical technique for protection of the articular fosse. J Craniofac Surg 2006; 17( 3): 523–527.
Batsakis JG, Rice DH . The Pathology of head and neck tumors: vasoformative tumors, part 9B. Head Neck Surg 1981; 3( 4): 326–339.
Acknowledgements
I would like to conclude this article by expressing my gratitude to Dr Kou Kayamori of the Section of Oral Pathology, Division of Oral Health Sciences, Graduate School, Tokyo.
Disclosures: The content of this paper was presented at the 182nd Kanto Regional Meeting of the Japanese Society of Oral and Maxillofacial Surgeons (December 2006, in Chiba).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Michi, Y., Suzuki, M., Kurohara, K. et al. A case of hemangiopericytoma of the soft palate with articulate disorder and dysphagia. Int J Oral Sci 5, 111–114 (2013). https://doi.org/10.1038/ijos.2013.25
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2013.25
Keywords
This article is cited by
-
Haemangiopericytoma/Solitary Fibrous Tumour of Mandible: An Uncommonness in the Oral Cavity
Journal of Maxillofacial and Oral Surgery (2021)
-
Hemangiopericytoma of Gingiva in a 4-Year-Old Child: A Rare Case Report
Journal of Maxillofacial and Oral Surgery (2019)