Abstract
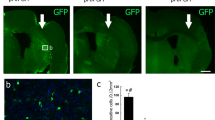
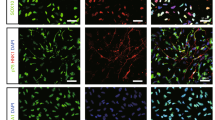
Recent studies have reported that glial cell line-derived growth factor (GDNF) has neurotrophic effects on the central nervous system, and the neural stem cells (NSCs) engrafted in animal models of stroke survive and ameliorate the neurological deficits. In this study, a stable human NSC line overexpressing GDNF (F3.GDNF) was transplanted next to the intracerebral hemorrhage (ICH) lesion site and a possible therapeutic effect was investigated. F3.GDNF human NSC line was transplanted into the cortex overlying the striatal ICH lesion. ICH was induced in adult mice by the unilateral injection of bacterial collagenase into the striatum. The animals were evaluated for 8 weeks with rotarod and limb placement tests. Transplanted NSCs were detected by β-gal immunostaining with double labeling of neurofilament, microtubule associated protein-2, glial fibrillary acidic protein or human nuclear matrix antigen (HuNuMA). F3.GDNF human NSCs produced a four times higher amount of GDNF over parental F3 cells in vitro, induced behavioral improvement in ICH mice after brain transplantation and two- to threefold increase in cell survival of transplanted NSCs at 2 and 8 weeks post-transplantation. In F3.GDNF-grafted ICH brain, a significant increase in the antiapoptotic protein and cell survival signal molecules, and a marked reduction in proapoptotic proteins were found as compared with control group. Brain transplantation of human NSCs overexpressing GDNF in ICH animals provided functional recovery in ICH animals, and survival and differentiation of grafted human NSCs. These results indicate that the F3.GDNF human NSCs should be of a great value as a cellular source for the cellular therapy in animal models of human neurological disorders including ICH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF . Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450–1460.
Inagawa T . What are the actual incidence and mortality rates of intracerebral hemorrhage? Neurosurg Rev 2002; 25: 237–246.
Gebel JM, Broderick JP . Intracerebral hemorrhage. Neurol Clin 2000; 18: 419–438.
NINDS ICH Workshop Participants. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 2005; 36: 23–41.
McKay R . Stem cells in the central nervous system. Science 1997; 276: 66–71.
Gage FH . Mammalian neural stem cells. Science 2000; 287: 1433–1438.
Lindvall O, Kokaia Z . Stem cells for treatment of neurological disorders. Nature 2006; 441: 1094–1096.
Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nature Biotech 1998; 16: 1033–1039.
Kim SU . Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 2004; 24: 159–171.
Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, Shizuki I et al. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res 2004; 78: 215–223.
Chu K, Kim M, Jeong SW, Kim SU, Yoon BW . Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett 2003; 343: 129–133.
Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH et al. Human neural stem cells improve sensorimotor deficits in the rat brain with experimental focal ischemia. Brain Res 2004; 1016: 145–153.
Chu K, Kim M, Chae SH, Jeong SW, Kang KS, Jung KH et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem cells in rats with focal cerebral ischemia. Neurosci Res 2004; 50: 459–465.
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC et al. Transplanted human fetal neural stem cells survive, migrate and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA 2004; 101: 11839–11844.
Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK . Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 2003; 34: 2258–2263.
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH et al. Brain transplantation of human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007; 25: 211–224.
Lee HJ, Kim KS, Park IH, Kim SU . Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One 2007; 156: 1–14.
Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 2008; 131: 616–629.
Abe K . Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cereb Blood Flow Metab 2000; 20: 1393–1408.
Chao MV . Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 2003; 4: 299–309.
Airaksinen MS, Saarma M . The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002; 3: 383–394.
Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomyinduced degeneration in the adult brain. Nature 1995; 373: 339–341.
Garcia-Martinez JM, Perez-Navarro E, Gavalda N, Alberch J . Glial cell line-derived neurotrophic factor promotes the arborization of cultured striatal neurons through the p42/p44 mitogen-activated protein kinase pathway. J Neurosci Res 2006; 28: 68–79.
Wang Y, Lin S-Z, Chiou A-L, Williams LR, Hoffer BJ . Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci 1997; 17: 4341–4348.
Kitagawa H, Sasaki C, Sakai K, Mori A, Mitsumoto Y, Mori T et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents ischemic brain injury after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 1999; 19: 1336–1344.
Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M . Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol Dis 2001; 8: 655–666.
Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M . HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Exp Neurol 2003; 183: 47–55.
Cheng H, Huang SS, Lin SM, Lin MJ, Chu YC, Chih CL et al. The neuroprotective effect of glial cell line-derived neurotrophic factor in fibrin glue against chronic focal cerebral ischemia in conscious rats. Brain Res 2005; 1033: 28–33.
Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O . Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 2006; 37: 2361–2367.
Kim SU, Stern J, Kim MW, Pleasure DE . Culture of purified rat astrocytes in serum-free medium supplemented with mitogen. Brain Res 1983; 274: 79–86.
Kim SU, Moretto G, Lee V, Yu RK . Neuroimmunology of gangliosides in human neurons and glial cells in culture. J Neurosci Res 1986; 15: 303–321.
Satoh JI, Kim SU . Differential expression of heat shock protein HSP27 in human neurons and glial cells in culture. J Neurosci Res 1995; 41: 805–818.
Nagai A, Hatori K, Kobayashi S, Kim SU . Erythropoietin and erythropoietin receptors in human neurons, astrocytes, microglia and oligodnedrocytes grown in culture. J Neuropath Exp Neurol 2001; 60: 386–392.
Del Bigio MR, Yan HJ, Buist R, Peeling J . Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 1996; 27: 2312–2320.
Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J . An alpha(2)- adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology 2001; 40: 597–606.
West MJ, Slomianka L, Gundersen HJ . Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991; 231: 482–497.
Paxinos G, Franklin K . The Rat Brain in Stereotaxic Coordinates. Academic Press: New York, 2003.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F . GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993; 260: 1130–1132.
Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994; 266: 1062–1064.
Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995; 373: 335–339.
Li L, Wu W, Lin LF, Lei M, Oppenheim RW, Houenou LJ . Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc Natl Acad Sci USA 1995; 92: 9771–9775.
Zhang WR, Sato K, Iwai M, Nagano I, Manabe Y, Abe K . Therapeutic time window of adenovirus-mediated GDNF gene transfer after transient middle cerebral artery occlusion in rat. Brain Res 2002; 23: 140–145.
Jin G, Omori N, Li F, Nagano I, Manabe Y, Shoji M et al. Protection against ischemic brain damage by GDNF affecting cell survival and death signals. Neurol Res 2003; 25: 249–253.
Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis J . Intravenous administration of GDNF neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 2006; 84: 1495–1504.
Acknowledgements
This study was supported by the Korean Ministry of Education, Science and Technology (21st Frontier Research program, M100-2000345) and the Canadian Myelin Research Initiative.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, H., Park, I., Kim, H. et al. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther 16, 1066–1076 (2009). https://doi.org/10.1038/gt.2009.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.51
Keywords
This article is cited by
-
Application of stem cells and exosomes in the treatment of intracerebral hemorrhage: an update
Stem Cell Research & Therapy (2022)
-
Neurogenesis in Stroke Recovery
Translational Stroke Research (2017)
-
Pretreatment of Mouse Neural Stem Cells with Carbon Monoxide-Releasing Molecule-2 Interferes with NF-κB p65 Signaling and Suppresses Iron Overload-Induced Apoptosis
Cellular and Molecular Neurobiology (2016)
-
Intracerebral hemorrhage in mouse models: therapeutic interventions and functional recovery
Metabolic Brain Disease (2015)
-
Transplantation of Neural Stem Cells That Overexpress Sod1 Enhances Amelioration of Intracerebral Hemorrhage in Mice
Journal of Cerebral Blood Flow & Metabolism (2014)