Abstract
Purpose
To describe pooled efficacy and safety data from two phase 3 studies comparing brinzolamide 1%/brimonidine 0.2% fixed combination (BBFC) with its component medications, brinzolamide and brimonidine, in patients with open-angle glaucoma or ocular hypertension.
Methods
Data were pooled from two nearly identical clinical trials comparing BBFC with its component medications, each given three times daily. The 3-month efficacy outcome was mean intraocular pressure (IOP) at 0800, 1000, 1500, and 1700 hours. Safety outcomes included adverse events (AEs), best-corrected visual acuity, examination of ocular structures, pachymetry, perimetry, and vital signs.
Results
A total of 1350 patients were enrolled and included in this analysis (BBFC, n=437; brinzolamide, n=458; brimonidine, n=455). Baseline mean IOP levels were similar among the three treatment groups. At 3 months, mean IOP of the BBFC group was significantly lower than that of either monotherapy group (P<0.0001) at all the four time points. A total of 272 patients (20.1%) experienced at least one treatment-related AE (BBFC, 24.6%; brinzolamide, 18.7%; brimonidine, 17.4%), the majority of which were ocular AEs. One serious AE, moderate intensity chest pain, was considered related to brinzolamide treatment and resulted in study discontinuation.
Conclusions
This analysis strengthens the conclusions drawn from the two individual phase 3 studies showing that, in patients with open-angle glaucoma or ocular hypertension, BBFC had significantly superior IOP-lowering activity compared with either brinzolamide or brimonidine alone and a safety profile consistent with that of its individual components.
Similar content being viewed by others
Introduction
Pharmacological therapy is the most common first-line approach for patients with glaucoma requiring intraocular pressure (IOP) reduction.1 More than one medication is necessary in many cases, with one study reporting that a 20% reduction in IOP to ≤24 mm Hg required two medications in 30% of patients and ≥3 medications in an additional 9%.2 Patients requiring two medications can either concomitantly administer two separate medications or use a single fixed-combination medication. Fixed-combination therapy may be preferred due to reduced exposure to ocular preservatives, avoidance of the potential for washout of the first medication by administration of the second, and increased patient convenience resulting from having only one bottle of medication, which could increase the likelihood of adherence to glaucoma therapy and potentially lower cost from fewer copays.3
Brinzolamide 1%/brimonidine 0.2% (BBFC) is an investigational fixed-combination therapy that contains a carbonic anhydrase inhibitor and an alpha agonist. All currently available fixed-combination therapies contain the beta blocker timolol. Although beta blockers are among the most commonly used ocular antihypertensive medications, they are contraindicated for patients with certain respiratory or cardiac conditions.4 Thus, BBFC will provide a non-beta blocker-containing fixed-combination alternative to current fixed-combination therapies for these patients.
Two phase 3 studies (C-10-033 and C-10-039) were recently conducted that assessed the efficacy and safety of BBFC using a nearly identical, randomized, 3-month, contribution-of-elements design. The only difference between the two trials was that the C-10-039 study collected additional safety information during a 3-month safety extension. The aim of the current analysis was to more robustly describe the efficacy and safety of BBFC by pooling the data from these two studies.
Materials and methods
Pooled analysis: study selection and data extraction
The data for this pooled analysis were obtained from two nearly identical, phase 3, randomized, 3-month clinical trials (Alcon studies C-10-033 and C-10-039) evaluating the safety and efficacy of BBFC using a contribution-of-elements design. The trials were identical in study design and eligibility criteria, which allowed the data to be pooled for analysis. The methodology of the two prior studies has been described previously.5, 6 Briefly, patients with open-angle glaucoma or ocular hypertension were randomly assigned to treatment for 3 months with BBFC, brinzolamide 1%, or brimonidine 0.2%. Patients demonstrated baseline IOP between 24 and 36 mm Hg at the 0800 hours time point and between 21 and 36 mm Hg at the 1000 hours time point, and they were seen at 2 weeks, 6 weeks, and 3 months, with IOP assessments at 0800, 1000, 1500, and 1700 hours at each visit. The only difference between the two studies was that C-10-039 had an additional 3-month safety extension (data will not be presented here). Efficacy and safety methods and results from each trial (through the 3-month visit for C-10-039) have been previously published.5, 6 Data obtained from each study for the present analysis included demographics, IOP at baseline, and at the 2-week, 6-week, and 3-month visits (at all the four time points), percentage and absolute changes in IOP from baseline for the 2-week, 6-week, and 3-month visits (at all four time points), solicited and unsolicited adverse events (AEs), best-corrected visual acuity (BCVA), slit-lamp biomicroscopy observations, pachymetry, automated perimetry, fundus parameters, and resting pulse and blood pressure.
Statistical methods
The pooled analysis was performed according to a predefined analysis plan. Pairwise comparisons of pooled mean IOP (BBFC vs brinzolamide and BBFC vs brimonidine) at each scheduled on-therapy study visit at all the four time points (0800, 1000, 1500, and 1700 hours) were based on the least squares means derived from a statistical model that accounts for correlated IOP measurements within patient. A two-sided α-level of 0.05 was used to declare statistical significance. The pooled intent-to-treat population was used for primary analysis. The pooled safety population, which included all patients from both the studies who received study medication, was used for safety analysis.
Descriptive statistics were calculated for all safety parameters (AEs, BCVA, slit-lamp biomicroscopy observations, pachymetry, automated perimetry, fundus parameters, and resting pulse rate and blood pressure) and for IOP, IOP change from baseline, and IOP percentage of change from baseline. Statistical analysis of the pooled data was performed using SAS (SAS Institute, Cary, NC, USA).
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Participant flow
A total of 660 patients from C-10-033 and 690 patients from C-10-039 were enrolled. Of these 1350 patients, 1209 patients (C-10-033, n=594; C-10-039, n=615) completed the 3-month visit. Patient disposition is described in Figure 1.
Demographics and baseline characteristics
Demographic and baseline characteristics were well balanced among the three arms (Table 1). Mean age of the intent-to-treat population was 64.7±10.5 years, 58.4% were women, and 71.0% had a diagnosis of open-angle glaucoma.
Intraocular pressure
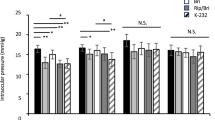
Baseline mean IOP levels were similar among the three treatment groups at each of the four time points (Table 2). For the 3-month primary end point, mean IOP of the BBFC group was significantly lower than that of either the brinzolamide group or the brimonidine group at each time point (P<0.0001; Table 2). For the 2-week and 6-week supportive end points, mean IOP of the BBFC group was significantly lower at all the time points than the mean IOP of either the brinzolamide group (P<0.0001) or the brimonidine group (P<0.0001; 2-week data not shown). At each visit, the BBFC group demonstrated the largest reduction in IOP from baseline at all the four time points compared with either monotherapy (Figure 2; 2-week data not shown). Reductions in the BBFC group across visits and time points ranged from 22.5 to 34.5% (5.5–8.9 mm Hg), 17.0 to 23.0% (4.2–5.9 mm Hg) in the brinzolamide group, and 13.4 to 26.9% in the brimonidine group (3.3–6.9 mm Hg).
Adverse events and other safety measures
A total of 272 (20.1%) patients experienced at least one treatment-related AE (BBFC group, n=107, 24.6%; brinzolamide group, n=86, 18.7%; brimonidine group, n=79, 17.4%), the majority of which were ocular AEs (Table 3). The brinzolamide-containing groups showed a higher incidence of blurred vision (5.3–6.5%) and dysgeusia (3.9–8.3%) compared with the brimonidine group (0.2% for both) and the brimonidine-containing groups showed a higher incidence of ocular hyperemia (2.1–3.3%), dry mouth (2.4–3.0%), and eye allergy (1.1–2.5%) compared with the brinzolamide group (0.7, 0, and 0%, respectively). Twenty patients experienced 29 serious AEs, of which 1 was judged by an investigator to be related to treatment. This was a case of chest pain of moderate intensity experienced by a patient in the brinzolamide group, which resulted in study discontinuation and subsequent resolution of the AE.
Seven of the treatment-related AEs were severe (BBFC group, one case each of allergic conjunctivitis, fatigue, blurred vision; brinzolamide group, two cases of blurred vision; brimonidine group, one case each of eye allergy and atopic dermatitis), three of which resulted in treatment discontinuation. In total, 87 patients discontinued participation due to treatment-related AEs (BBFC group, n=43, 9.9%; brinzolamide group, n=10, 2.2%; brimonidine group, n=34, 7.5%).
From the baseline visit to the 3-month visit, the change in mean number of letters read was <1 letter in all the groups. Of the five patients observed to have a decrease in BCVA, one case was judged to be related to BBFC treatment, but the reduced visual acuity was mild in severity and resolved without treatment. Using slit-lamp biomicroscopy, investigators observed ≥1-unit increases from the baseline visit to the exit visit (last on-therapy visit up to and including 3-month visit) for evidence of inflammation or significant structural changes in or discharge from eyelids/conjunctiva in 9.9% (43 of 433) of the BBFC group, 3.3% (15 of 458) of the brinzolamide group, and 8.7% (39 of 450) of the brimonidine group. No other significant changes were noted in visual acuity, anterior or posterior segment examination, pachymetry, or perimetry.
A slight trend towards a decrease in both systolic and diastolic mean blood pressure was observed from the baseline visit to the 3-month visit at the 1000 hours time point for patients from the BBFC group (5.3 mm Hg systolic decrease and 2.8 mm Hg diastolic decrease) and the brimonidine group (3.8 mm Hg systolic decrease and 1.9 mm Hg diastolic decrease). For the brinzolamide group, systolic and diastolic mean blood pressure remained within 2 mm Hg of the mean baseline levels at all the visits. Two patients from the BBFC group had a blood pressure decrease coded as an AE. No patient experienced a clinically meaningful decrease in pulse rate.
Discussion
Based on this pooled analysis of patients from the two phase 3 BBFC clinical trials, we conclude that BBFC has significantly superior IOP-lowering activity compared with either brinzolamide 1% or brimonidine 0.2% in patients with open-angle glaucoma or ocular hypertension while providing a safety profile consistent with that of its individual components. These conclusions are consistent with the findings of the individual phase 3 studies.5, 6 In addition, the pooled analysis showed that fewer patients in the BBFC group (n=2) discontinued due to a lack of IOP control than in either the brinzolamide group (n=8) or the brimonidine group (n=15). The magnitude of peak IOP reduction from baseline observed with BBFC (33.4–34.5%) is consistent with those of other fixed-combination therapies, such as dorzolamide 2%/timolol 0.5% (26.1–32.3%)7, 8, 9 and brimonidine 0.2%/timolol 0.5% (32.3%).9 Taken together, these data suggest that BBFC provides consistent diurnal IOP control (>5.5 mm Hg reduction) that is superior to either component through 3 months of treatment.
Due to its greater sample size, the pooled analysis improved the robustness of the safety results relative to those of the individual studies, permitting the potential identification of uncommon AEs. Despite this, the pooled analysis failed to identify any novel safety concerns, thus supporting the safety conclusion of the individual studies,5, 6 which was that the safety profile of BBFC was consistent with that of the individual components.10, 11 The incidence of treatment-related AEs was slightly higher in the BBFC group than in the brinzolamide or brimonidine groups (24.6 vs 18.7 or 17.4%, respectively), as was the incidence of treatment-related AEs leading to discontinuation (9.9 vs 2.2 or 7.5%, respectively). No treatment-related serious AEs were experienced by any patients taking BBFC.
As with the individual BBFC phase 3 trials, the BBFC and brimonidine groups in the pooled analysis exhibited modest, clinically insignificant reductions in cardiac parameters, in accordance with the expected safety profile of brimonidine.10
BBFC is unique among fixed combinations. All other commercially available fixed-combination therapies contain a beta blocker in combination with another IOP-lowering drug. Beta blockers are associated with serious side effects, such as severe respiratory and cardiac reactions.12, 13 Therefore, all current fixed-combination therapies are contraindicated for patients with certain respiratory or cardiac conditions, including patients with asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second- or third-degree atrioventricular block, cardiogenic shock, or overt cardiac failure.4 A study of nearly 26 000 veterans with glaucoma revealed that 23% also had reactive airways disease (an umbrella term encompassing bronchitis, emphysema, asthma, and COPD diagnoses) and 12% had congestive heart failure.14 This represents a substantial portion of an elderly population (median age of 80 years)14 in whom beta blocker use may be inappropriate. Furthermore, in that study, 88% of patients with reactive airways disease were dispensed glaucoma medications (primarily beta blockers) that had the potential to aggravate bronchoconstriction. In addition to serious respiratory and cardiac concerns, beta blockers are also associated with altered mental status,13 which may be insidious and difficult to identify. All of this information taken together demonstrates the unmet need for patients to have a fixed-combination glaucoma medication that does not contain a beta blocker.
This analysis has some limitations. The short-term, 3-month end point of the studies prevents conclusions to be drawn regarding the long-term safety of BBFC. In the future, the 6-month safety results from C-10-039 will be published, providing additional information regarding these AEs. However, neither this nor the 6-month safety analysis of C-10-039 is capable of fully characterizing the allergy-related AEs associated with brimonidine use. Finally, the contribution-of-elements design of the two studies provides no information regarding how BBFC compares with other fixed-combination therapies in a clinical setting, which is an important consideration for physicians when making treatment decisions.
This analysis strengthens the conclusions drawn from the two individual phase 3 studies of BBFC—that this fixed-combination therapy has significantly superior IOP-lowering activity compared with either brinzolamide 1% alone or brimonidine 0.2% alone in patients with open-angle glaucoma or ocular hypertension. It also suggests that BBFC has a safety profile consistent with that of its individual components.

References
American Academy of Ophthalmology. Primary Open-Angle Glaucoma Suspect, Preferred Practice Pattern. American Academy of Ophthalmology: San Francisco, CA, USA, 2005 Available at http://www.aao.org/ppp.
Kass MA, Heuer DK, Higginbotham EJ, Heuer DK, Higginbotham EJ, Johnson CA et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–713.
Fechtner RD, Realini T . Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol 2004; 15: 132–135.
Timolol maleate ophthalmic solution [package insert]: Falcon Pharmaceuticals, Ltd.: Fort Worth, TX, USA, 2004.
Nguyen QH, McMenemy MG, Realini T, Whitson JT, Goode SG . Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%. J Ocul Pharmacol Ther 2013; 29: 290–297.
Katz G, DuBiner H, Samples J, Vold S, Sall K . Three-month randomized trial of fixed-combination brinzolamide 1%/brimonidine 0.2%. JAMA Ophthalmol 2013 e-pub ahead of print 11 April 2013; doi: 10.1001/jamaophthalmol.2013.188.
Fechtner RD, Airaksinen PJ, Getson AJ, Lines CR, Adamsons IA . Efficacy and tolerability of the dorzolamide 2%/timolol 0.5% combination (COSOPT) versus 0.005% (XALATAN) in the treatment of ocular hypertension or glaucoma: results from two randomized clinical trials. Acta Ophthalmol Scand 2004; 82: 42–48.
Nixon DR, Yan DB, Chartrand JP, Piemontesi RL, Simonyi S, Hollander DA . Three-month, randomized, parallel-group comparison of brimonidine-timolol versus dorzolamide-timolol fixed-combination therapy. Curr Med Res Opin 2009; 25: 1645–1653.
Shin DH, Feldman RM, Sheu WP . Efficacy and safety of the fixed combinations latanoprost/timolol versus dorzolamide/timolol in patients with elevated intraocular pressure. Ophthalmology 2004; 111: 276–282.
Alphagan P [package insert]. Allergan, Inc.: Irvine, CA, USA. 2008.
Azopt [package insert]. Alcon Laboratories, Inc.: Fort Worth, TX, USA, 2008.
Nordlund JR, Pasquale LR, Robin AL, Rudikoff MT, Ordman J, Chen KS et al. The cardiovascular, pulmonary, and ocular hypotensive effects of 0.2% brimonidine. Arch Ophthalmol 1995; 113: 77–83.
Stewart WC, Garrison PM . Beta-blocker-induced complications and the patient with glaucoma. Newer treatments to help reduce systemic adverse events. Arch Intern Med 1998; 158: 221–226.
Roughead EE, Kalisch LM, Pratt NL et al. Managing glaucoma in those with co-morbidity: not as easy as it seems. Ophthalmic Epidemiol 2012; 19: 74–82.
Acknowledgements
This pooled analysis was funded by Alcon Research, Ltd., as was the medical writing assistance, which was provided by Jennifer Klem, PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TR is a consultant to Alcon and is on the speaker’s bureau for Lumenis. QHN is on the speaker’s bureau for Alcon, Allergan, and Merck. GK is a consultant to Alcon and is on the speaker’s bureau for Alcon. HDB is on the speaker’s bureau for Alcon.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Realini, T., Nguyen, Q., Katz, G. et al. Fixed-combination brinzolamide 1%/brimonidine 0.2% vs monotherapy with brinzolamide or brimonidine in patients with open-angle glaucoma or ocular hypertension: results of a pooled analysis of two phase 3 studies. Eye 27, 841–847 (2013). https://doi.org/10.1038/eye.2013.83
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.83
Keywords
This article is cited by
-
Efficacy and safety of fixed-combination brimonidine tartrate/timolol maleate in primary open-angle glaucoma, including normal-tension glaucoma
Japanese Journal of Ophthalmology (2021)
-
Real-life experience of using brinzolamide/brimonidine fixed drop combination in a tertiary glaucoma centre
International Ophthalmology (2020)
-
Brinzolamide/Brimonidine: A Review of Its Use in Patients with Open-Angle Glaucoma or Ocular Hypertension
Drugs & Aging (2015)
-
Randomized Trial of Brinzolamide/Brimonidine Versus Brinzolamide Plus Brimonidine for Open-Angle Glaucoma or Ocular Hypertension
Advances in Therapy (2014)