Abstract
The eukaryotic cell responds to various forms of environmental stress by adjusting the rates of mRNA translation thus facilitating adaptation to the assaulting stress. One of the major pathways that control protein synthesis involves the phosphorylation of the α-subunit of eukaryotic initiation factor eIF2 at serine 51. Different forms of DNA damage were shown to induce eIF2α phosphorylation by using PERK, GCN2 or PKR. However, the specificity of the eIF2α kinases and the biological role of eIF2α phosphorylation pathway in the DNA damage response (DDR) induced by chemotherapeutics are not known. Herein, we show that PKR is the eIF2α kinase that responds to DDR induced by doxorubicin. We show that activation of PKR integrates two signaling pathways with opposing biological outcomes. More specifically, induction of eIF2α phosphorylation has a cytoprotective role, whereas activation of c-jun N-terminal kinase (JNK) by PKR promotes cell death in response to doxorubicin. We further show that the proapoptotic effects of JNK activation prevail over the cytoprotection mediated by eIF2α phosphorylation. These findings reveal that PKR can be an important inducer of cell death in response to chemotherapies through its ability to act independently of eIF2α phosphorylation.
Similar content being viewed by others
Main
The eukaryotic cell has established intricate mechanisms to cope with environmental stress. It does so by changing protein expression in a manner that promotes adaptation to the assaulting stress. Protein expression within the cell is regulated at the transcriptional, translational and post-translational levels. Translational control is often used under conditions of cellular stress because it allows immediate and selective changes in protein levels.1 Translation itself is divided into three distinct phases: initiation, elongation and termination. By far the most explored step is that of initiation, which has been shown to be regulated by different extracellular stimuli.1 One of the major pathways that control translation initiation is that of the phosphorylation of the α-subunit of translation initiation factor eIF2.2 Phosphorylation of eIF2α at serine 51 (S51) leads to the inhibition of global protein synthesis providing the cell with the opportunity to elicit adaptive responses not only by saving energy but also by preventing the accumulation of unwanted proteins that could interfere with cellular functions.1
There are four eIF2α kinases that share a homologous kinase domain (KD) but possess different regulatory domains that enable them to become activated by distinct stimuli.2 The eIF2α kinases are the heme-regulated inhibitor, which is found mainly in erythroid cells and is activated by heme deficiency; the endoplasmic reticulum (ER)-resident kinase (PERK), which is activated by ER stress and inhibits protein synthesis as part of the unfolded protein response; the general amino-acid nonderepressing kinase 2 (GCN2), which responds to amino-acid deprivation and becomes activated by uncharged tRNA and the RNA-dependent protein kinase PKR, an interferon-inducible protein that becomes activated by double-stranded (ds) RNA.2 Previous work suggested that induction of eIF2α phosphorylation can be either cytoprotective or proapoptotic. That is, transient induction of eIF2α phosphorylation functions mostly cytoprotectively through the activation of pathways that promote cell survival such as the phosphatidylinositol-3 kinase (PI3K)3 and nuclear factor-κB (NF-κB) pathways.4, 5 However, prolonged induction of eIF2α phosphorylation is mainly proapoptotic.5, 6 Although eIF2α phosphorylation has a major role in mediating the biological effects of the eIF2α kinases, their ability to function independently of eIF2α phosphorylation has been reported.7, 8
Previous findings provided evidence that eIF2α phosphorylation is induced by genotoxic stress. That is, GCN2, PERK and PKR have been implicated in the DNA damage response (DDR) by various stimuli.9, 10, 11, 12 However, the specificity of the eIF2α kinases and the role of eIF2α phosphorylation in DDR induced by chemotherapeutics have not been elucidated. Herein, we show that PKR is specifically activated by doxorubicin leading to the induction of eIF2α phosphorylation and c-jun N-terminal kinase (JNK) activity. We also show that eIF2α phosphorylation conveys a cytoprotective effect, which is counteracted by JNK activation, the latter being required for PKR-mediated cell death. These data reveal a dual function for PKR in response to DNA damage with potential implications in treatments with chemotherapeutic drugs.
Results
PKR promotes cell death in response to doxorubicin treatment
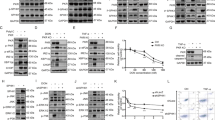
Previous work from our group showed that PKR mediates the induction of G1 arrest by enhancing the activation of the tumor suppressor p53 and is involved in p53 phosphorylation at serine 18 in mouse embryonic fibroblasts (MEFs) subjected to DNA damage.13 We examined the biological role of PKR in response to DNA damage in MEFs that are deficient in p53 due to spontaneous immortalization. For this purpose immortalized MEFs completely deficient in PKR activity (PKR−/− MEFs)14, 15 together with their isogenic wild-type counterparts were treated with the chemotherapeutic drug doxorubicin and subjected to analysis of cell death by flow cytometry. We noticed that PKR was required for optimal induction of cell death in response to doxorubicin (Figure 1a). The higher sensitivity of PKR+/+ MEFs to the cytotoxic effects of doxorubicin was supported by the higher levels of cleaved caspase-3 in these cells compared with PKR−/− MEFs for the various periods of treatment (Figure 1b). Moreover doxorubicin treatment did not cause differences in cell-cycle arrest between PKR+/+ and PKR−/− MEFs (Supplementary Figure S1) indicating a specific role of PKR in the induction of cell death.
PKR promotes doxorubicin-induced cell death. (a) PKR+/+ and PKR−/− MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods. Cells were subjected to FACS analysis after propidium iodide staining. Cell death is represented by the percentage (%) of cells in sub-G1. Histograms represent the mean cell death from five independent experiments after subtraction of background cell death (untreated control cells) (n=5). Statistical significance of the difference as calculated by Student's t-test is with *P<0.0003, **P<0.0009. (b) PKR+/+ and PKR−/− MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods. Protein extracts (50 μg) were subjected to western blot analysis for cleaved caspase-3 (a) and actin (b). Histograms represent the mean value of the ratio of cleaved caspase-3 to actin after normalization to that of lane 4 for the indicated lanes of the western blot from five independent experiments (n=5). *P<0.005, **P<0.006
Because both PERK and GCN2 have been shown to respond to DNA damage, we next wanted to examine the specificity of eIF2α kinases to doxorubicin treatment. To this end, we treated PERK−/− and GCN2−/− MEFs as well as their isogenic wild-type MEFs with doxorubicin and subjected to fluorescence-activated cell sorting (FACS) analysis. We observed that cell death was equally induced in PERK+/+ and PERK−/− as well as in GCN2+/+ and GCN2−/− MEFs thus excluding a role for either eIF2α kinase in promoting the apoptotic effects of doxorubicin (Figure 2). Moreover, we did not observe any differences in cell-cycle arrest induced by the drug between PERK+/+ and PERK−/− MEFs, GCN2+/+ and GCN2−/− MEFs (data not shown). Taken together, these data suggested that PKR specifically responds to DNA damage caused by doxorubicin leading to the induction of cell death.
PERK and GCN2 do not contribute to doxorubicin-induced cell death. PERK+/+ and PERK−/− MEFs (a) or GCN2+/+ and GCN2−/− MEFs (b) were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods and subjected to FACS analysis after propidium iodide staining. Cell death is represented by the percentage (%) of cells in sub-G1. Histograms represent the mean cell death from three independent experiments after subtraction of background cell death (untreated control cells) (n=3)
Phosphorylation of eIF2α in response to doxorubicin treatment is mediated by PKR and exerts a cytoprotective role
Next, we determined the role of eIF2α phosphorylation in doxorubicin-mediated cell death. We first examined the induction of eIF2α phosphorylation at S51 in PKR−/− MEFs together with their isogenic wild-type counterparts on doxorubicin treatment. We observed an induction of eIF2α phosphorylation in PKR+/+ but not in PKR−/− MEFs (Figure 3, panel a). Contrary to this, eIF2α phosphorylation was similarly induced in PERK−/− MEFs (panel c) or GCN2−/− MEFs (panel e) compared with their corresponding isogenic wild-type MEFs. Induction of eIF2α phosphorylation by doxorubicin resulted in the upregulation of activating transcription factor 4 (ATF4) in PKR+/+ but not in PKR−/− MEFs (Supplementary Figure S2A), supporting a functional role of eIF2α phosphorylation in this process. Moreover, induction of eIF2α phosphorylation by doxorubicin occurred in various human tumor cells (Supplementary Figure S2B) concomitantly with an increased PKR autophosphorylation at T446 (Supplementary Figure S2B, panel c) confirming the activation of PKR by doxorubicin in human cells.
PKR induces eIF2α phosphorylation in response to doxorubicin treatment. The indicated types of MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods. Protein extracts (50 μg) were subjected to immunoblot analysis for phosphorylated eIF2α (a, c, e) and total eIF2α (b, d, f). Histograms represent the mean value of the ratio of eIF2α phosphorylation levels to total eIF2α levels for the treated cells normalized to that of their untreated control from six independent experiments for PKR (n=6), five independent experiments for PERK (n=5) and five independent experiments for GCN2 (n=5). Statistical significance of the difference as calculated by Student's t-test is with *P<0.05, **P<0.02
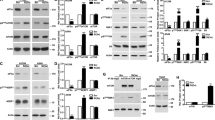
To understand the biological effects of eIF2α phosphorylation, we used MEFs containing either a wild-type allele of eIF2α (i.e., eIF2αS/S) or an allele bearing the S51A mutation (i.e., eIF2αA/A MEFs). First, we verified that doxorubicin treatment induced eIF2α phosphorylation at S51 in eIF2αS/S MEFs (Figure 4a). Next, we evaluated cell death in eIF2αS/S and eIF2αA/A MEFs by flow cytometry. We observed that eIF2αA/A MEFs were more susceptible than eIF2αS/S MEFs to cell death by doxorubicin (Figure 4b). Also, the higher sensitivity of eIF2αA/A MEFs to the proapoptotic effects of doxorubicin was further indicated by the increased expression of cleaved caspase-3 in these cells compared with eIF2αS/S cells after doxorubicin treatment (Figure 4c). These data supported the notion that eIF2α phosphorylation conveys a cytoprotective effect on doxorubicin-treated MEFs and that PKR-mediated cell death by doxorubicin uses a pathway other than that of eIF2α phosphorylation.
Phosphorylation of eIF2α protects cells from doxorubicin-induced death. (a) eIF2αS/S and eIF2αA/A MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods. Protein extracts (50 μg) were subjected to immunoblotting for phosphorylated eIF2α (a) and total eIF2α (b). Histograms represent the mean value of the ratio of eIF2α phosphorylation levels to eIF2α total levels for the treated cells normalized to that of their untreated control from four independent experiments (n=4) for the eIF2αS/S MEFs. (b) eIF2αS/S and eIF2αA/A MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated time periods and subjected to FACS analysis after propidium iodide staining. Cell death is represented by the percentage (%) of cells in sub-G1. Histograms represent the mean cell death from five independent experiments after subtraction of background cell death (untreated control cells) (n=5). Statistical significance of the difference as calculated by Student's t-test is with *P<0.002, **P<0.02. (c) eIF2αS/S and eIF2αA/A MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated times. Protein extracts (50 μg) were subjected to western blot analysis for cleaved caspase-3 (a) and actin (b). Histograms represent the mean value of the ratio of cleaved caspase-3 to actin after normalization to the ratio of lane 2 for the indicated lanes from four independent experiments (n=4). Statistical significance of the difference as calculated by Student's t-test is with *P<0.006, **P<0.04
PKR induces JNK activity in response to doxorubicin treatment
Previous data established a role of stress-activated mitogen-activated protein kinases (MAPKs; i.e., JNK1/2, p38 and extracellular signal-regulated kinase (ERK) 1/2) in the regulation of cell death by DNA-damaging drugs including doxorubicin.16 Moreover, PKR was implicated in the activation of the p38 MAPK in response to dsRNA independently of eIF2α phosphorylation.17 These findings prompted us to examine a possible role of MAPKs in PKR-mediated cell death by doxorubicin. We observed that doxorubicin treatment resulted in a higher induction of JNK1/2 activity in PKR+/+ than in PKR−/− MEFs (Figure 5a, panel a). PKR specifically mediated the induction of JNK1/2 activity given that neither ERK1/2 nor p38 MAPK phosphorylation was significantly induced in PKR+/+ and PKR−/− MEFs (Figure 5a, panels c and e). Furthermore, JNK1/2 activation in PKR+/+ MEFs was maintained for long periods of doxorubicin treatment (Figure 5b) and was independent of eIF2α phosphorylation as JNK1/2 phosphorylation was similarly induced in eIF2αS/S and eIF2αA/A MEFs in response to doxorubicin (Figure 5c). To verify the ability of PKR to mediate JNK activation, we used human HT1080 cells expressing a conditionally active from of PKR, which is activated after treatment of cells with the antibiotic coumermycin.6 We found that conditional activation of PKR, which was assessed by the eIF2α phosphorylation, led to JNK1 activation by phosphorylation as detected by immunoblotting with phospho-specific antibodies (Supplementary Figure S3). Taken together, these findings showed the ability of PKR to induce JNK activity in mouse and human cells in response to doxorubicin.
PKR induces JNK phosphorylation in response to doxorubicin. (a) PKR+/+ and PKR−/− MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated times. Protein extracts (50 μg) were subjected to immunoblotting for phosphorylated JNK (a), total JNK1 (b), phosphorylated ERK (c), total ERK (d), phosphorylated p38 (e), total p38 (f). Histograms represent the mean value of the ratio of the phosphorylated protein levels to their total levels for the treated cells normalized to that of their untreated control from four independent experiments for JNK (n=4), four independent experiments for ERK (n=4) and four independent experiments for p38 (n=4). Statistical significance of the difference as calculated by Student's t-test is with *P<0.03. (b) PKR+/+ and PKR−/− MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated times. Protein extracts (50 μg) were subjected to immunoblotting for phosphorylated JNK (a) and total JNK1 (b). Histograms represent the mean value of the ratio of the phosphorylated protein levels to their total levels for the treated cells normalized to that of their untreated control from six independent experiments (n=6). Statistical significance of the difference as calculated by Student's t-test is with *P<0.02, **P<0.002. (c) eIF2αS/S and eIF2αA/A MEFs were left untreated (con) or treated with 1 μM doxorubicin (dox) for the indicated times. Protein extracts (50 μg) were subjected to immunoblotting for phosphorylated JNK (a) and total JNK1 (b). Histograms represent the mean value of the ratio of the phosphorylated protein levels to their total levels for the treated cells normalized to that of their untreated control from six independent experiments (n=6)
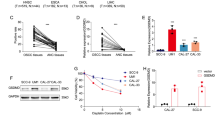
To determine the biological significance of JNK activation, we examined whether pharmacological inhibition of JNK1/2 had any effect on PKR-mediated cell death. To this end, we treated PKR+/+ and PKR−/− MEFs with doxorubicin in the absence or presence of the specific JNK inhibitor SP600125, and assessed cell death by FACS analysis (Figure 6). We observed that SP600125 alone had a minimal cytotoxic effect on both PKR+/+ and PKR−/− MEFs. However in combination with doxorubicin, SP600125 completely blocked the death of PKR+/+ MEFs and had a modest inhibitory effect on the death of PKR−/− MEFs (Figure 6). Collectively, these data suggested that induction of PKR-mediated cell death by doxorubicin requires the activation of JNK1/2.
Pharmacological inhibition of JNK blocks PKR-mediated cell death induced by doxorubicin. PKR+/+ and PKR−/− MEFs were treated with 10 μM SP600125 (SP600125), 1 μM doxorubicin (dox) or both drugs (dox+SP600125) for the indicated time periods and subjected to FACS analysis using propidium iodide staining. Control cells and doxorubicin-treated cells received the equivalent amount of DMSO in which SP600125 was dissolved. DMSO or SP600125 was added an hour before doxorubicin. Cell death is represented by the percentage (%) of cells in sub-G1. Histograms represent the mean cell death from three independent experiments (n=3). Group statistical significance of the differences as calculated by ANOVA is with *P<0.0001
Discussion
Our work shows that PKR can function as a mediator of cell survival as well as cell death in response to doxorubicin treatment. The prosurvival properties of PKR which are mediated by the induction of eIF2α phosphorylation, are counteracted by the proapoptotic effects arbitrated by JNK activation (Figure 7). Doxorubicin is a chemotherapeutic drug that belongs to the family of anthracyclines, which are widely used for the treatment of various forms of cancer. Doxorubicin induces a DDR through its ability to intercalate with DNA, generates reactive oxygen species and inhibits topoisomerase II activity.18 Inhibition of topoisomerase II is responsible for the production of ds breaks on DNA, which are recognized by proteins involved in DNA repair as well as proteins that control cell proliferation and cell death.19
A model for PKR function in response to doxorubicin. Activation of PKR by doxorubicin leads to the induction of eIF2α phosphorylation, which exerts a cytoprotective effect. Induction of PKR activity also leads to the activation of JNK, which promotes cell death. JNK activation proceeds independently of eIF2α phosphorylation and is sufficient to overcome the cytoprotective effects of eIF2α phosphorylation in immortalized MEFs
A major pathway activated by DDR involves the ataxia-telangiectasia mutated (ATM) kinase and the DNA-dependent protein kinase (DNA-PK), both of which are implicated in the induction of the DNA repair.19 ATM is also important in the induction of the G1 checkpoint as a result of the activation of the tumor suppressor p53.20 Previous work from our group showed that PKR is implicated in the phosphorylation and activation of p53 in response to DNA damage. More specifically, using PKR+/+ and PKR−/− MEFs expressing a temperature-sensitive mutant of p53 (tsp53), we showed that PKR promotes G1 arrest under conditions that tsp53 attains a wild-type conformation.13 We further showed that PKR phosphorylates p53 at serine 392 in vitro 21 and facilitates the phosphorylation of the tsp53 at serine 18 in response to ionizing irradiation or doxorubicin treatment.13 These data showed that in cells with functional p53, activation of PKR in response to doxorubicin and other types of DNA damage conveys a cytoprotective role by promoting G1 arrest. Here, we show an alternative function of PKR, which is the induction of cell death in MEFs with deficient p53 caused by spontaneous immortalization (data not shown). Our previous findings implied a possible interplay between PKR and ATM/DNA-PK. Nevertheless, PKR is unlikely to act upstream of ATM or DNA-PK given that phosphorylation of histone 2AX (H2AX) at serine 139 was efficiently induced in both PKR+/+ and PKR−/− MEFs after doxorubicin treatment (Supplementary Figure S4A). Furthermore, pharmacological inhibition of ATM and DNA-PK by wortmannin or ATM alone by KU55933 in combination with doxorubicin increased the index of death similarly in PKR+/+ and PKR−/− MEFs (Supplementary Figure S4B and S4C). Taken together, these results do not support a functional interplay between PKR and ATM and/or DNA-PK in response to doxorubicin.
Previous work by many researchers established a temporal role of eIF2α phosphorylation in the induction of cell death. That is, short-term induction of eIF2α phosphorylation leads to cytoprotection, which is mediated by the induction of prosurvival pathways such as the PI3K3 and NF-κB pathway.4 However, long-term induction of eIF2α phosphorylation promotes apoptosis through, at least in part, the activation of ATF4 – CCAAT/enhancer binding homologous protein pathway.2 However, there has been strong evidence to suggest that the signaling properties of eIF2α kinases in mammalian cells are not exclusively linked to the induction of eIF2α phosphorylation. For example, in contrast to DNA damage, activation of PKR or PERK in response to dsRNA or ER stress respectively decreases p53 levels through a mechanism that involves the activation of glycogen synthase kinase 3-β and proteasomal degradation of the tumor suppressor protein independently of eIF2α phosphorylation.22, 23, 24 Also, the antiviral effects of the eIF2α kinases are not always related to the inhibition of viral protein synthesis due to eIF2α phosphorylation. Specifically, the ability of PERK and GCN2 to impair vesicular stomatitis virus replication in MEFs does not require eIF2α phosphorylation.7 How the eIF2α kinases mediate their effects independently of eIF2α phosphorylation is not presently clear. One possibility is that proteins other than eIF2α can become substrates of the eIF2α kinases as has been shown for p5321 and nuclear factors associated with dsRNA proteins.25
Our work shows that PKR functions upstream of JNK in cells treated with doxorubicin. JNK is a well-established inducer of apoptosis through different mechanisms that involve both nuclear and cytoplasmic events.26 It has been shown that in response to doxorubicin, JNK activation promotes the nuclear localization of c-Abl, which in turn can induce cell death through the tumor suppressor p73.27 Conceivably, it is possible that PKR induces the activity of kinases that act upstream of JNK1/2, based on a previous study showing that activation of p38/MAPK by PKR is mediated by MAPK kinase kinase 6 in cells treated with dsRNA.17 It is also of interest that PKR physically associates with the apoptosis signal-regulating kinase 1 (ASK1) and promotes ASK1-mediated apoptosis induced by serum deprivation.28 ASK1 functions upstream of JNK,29 thus providing a tentative link between PKR and JNK activation. It is not presently known how PKR becomes activated by doxorubicin. DNA damage by doxorubicin might promote the interaction of PKR with proteins that function as activators. Another possibility may be that PKR is subjected to post-translational modifications in doxorubicin-treated cells, such as phosphorylation by other kinases and/or dephosphorylation by specific phosphatases, which induce its catalytic activity.
The ability of PKR to respond to doxorubicin treatment could have potential implications in cancer therapies. For example, it was previously shown that human invasive ductal breast carcinomas and several human breast tumor cell lines contain elevated levels of PKR compared with non-malignant breast cells.30, 31 However, the PKR-eIF2α phosphorylation arm was found to be compromised in malignant breast tumor cells due to upregulation of the guanine nucleotide exchange factor eIF2B,31 which antagonizes the inhibitory effects of phosphorylated eIF2α on protein synthesis.32 Interestingly, increased eIF2B levels were observed in various transformed cells and accounted for the high tolerance of these cells to inhibition of protein synthesis by phosphorylated eIF2α.33 Based on our findings, the possibility remains that breast tumors and possibly other types of tumors with increased levels of PKR are more susceptible to doxorubicin than tumors with low levels of the kinase. However, such an increased susceptibility could be counteracted by the cytoprotective effects of induced eIF2α phosphorylation after drug treatment. As such, doxorubicin in combination with compounds designed to impair eIF2α phosphorylation (e.g., eIF2α pseudosubstrate peptide-mimetic drugs) might prove to be effective means for the treatment of specific types of cancers with elevated PKR. Nevertheless, the therapeutic potential of PKR may also depend on the type of tumor based on evidence that PKR activation by chemotherapeutic drugs is proapoptotic for some types of tumors34 and cytoprotective for others.35
Materials and Methods
Cell culture and treatments
PKR−/− MEFs and their isogenic wild-type counterparts14 were grown in Dulbecco's modified Eagle's medium (DMEM; Wisent, St-Bruno, QC, Canada) supplemented with 10% fetal bovine serum (Wisent) and 100 U/ml of penicillin–streptomycin (Wisent). Isogenic wild-type and PERK−/− MEFs36 were grown in DMEM supplemented with 10% heat-inactivated bovine serum (Wisent) and 100 U/ml of penicillin–streptomycin. Isogenic wild-type and GCN2−/− MEFs37 were grown in DMEM and supplemented with 10% fetal bovine serum and 100 U/ml of penicillin-streptomycin plus 1 × nonessential amino acids (Invitrogen, Carlsbad, CA, USA). Isogenic eIF2αS/S and eIF2αA/A MEFs38 were grown in DMEM supplemented with 10% non-heat-inactivated bovine serum (Wisent) and 100 U/ml of penicillin–streptomycin plus 1 × nonessential amino acids and 1 × essential amino acids (Invitrogen). HT1080, H1299 and A549 cells were grown in DMEM supplemented with 10% heat-inactivated bovine serum (Wisent) and 100 U/ml of penicillin–streptomycin. Cells were treated with 1 μM of doxorubicin hydrochloride (Sigma, Oakville, ON, Canada) dissolved in water as well as 10 μM SP600125 (Calbiochem, Burlington, ON, Canada), 30 μM wortmannin (Bioshop, Burlington, ON, Canada) or 2 μM of KU55933 (Tocris Biosciences, Ellisville, MO, USA) dissolved in dimethyl sulfoxide (DMSO).
Protein extraction and immunoblotting
Protein extraction and quantification was performed as described elsewhere.6 For immunoblotting of caspase-3, we prepared extracts as previously described in Cheong et al.39 Immunoblotting of the rest of proteins was performed as previously described.6 The primary antibodies used are: rabbit polyclonal phospho-specific against S51 of eIF2α (Invitrogen), mouse monoclonal to eIF2α (Cell Signaling, Danvers, MA, USA), rabbit polyclonal against cleaved caspase-3 (Cell signaling), mouse monoclonal to actin (MP Biomedicals, Solon, OH, USA), rabbit polyclonal phospho-specific against T183/Y185 JNK1/2 (Cell Signaling), rabbit polyclonal to JNK1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal phospho-specific against T202/Y204 of ERK1/2 (Cell Signaling), rabbit polyclonal to ERK1/2 (Cell Signaling), rabbit polyclonal phospho-specific against T180/Y182 of p38 (Cell Signaling), rabbit polyclonal to p38 (Cell Signaling), rabbit polyclonal to γH2AX (Upstate Biotechnology, Temecula, CA, USA), rabbit polyclonal against H2AX (Thermo Scientific, Rockford, IL, USA), rabbit monoclonal phospho-specific to T446 of PKR (Abcam, Cambridge, MA, USA), mouse monoclonal against PKR (F9),40 rabbit polyclonal against ATF4 (Proteintech Group, Chicago, IL, USA), mouse monoclonal to GyrB (John Innes Enterprises, Norwich, UK). All antibodies were used at a concentration of 0.1–1 μg/ml. After incubation with horseradish-peroxidase-conjugated anti-mouse or anti-rabbit IgG (1 : 1000 dilution; KPL, Gaithersburg, MD, USA), proteins were visualized with the enhanced chemiluminescence detection system according to the manufacturer's protocol (PerkinElmer Life Sciences, Waltham, CA, USA). Quantification of the bands was performed by densitometry using the Scion Image software (Frederick, Maryland, USA).
Cell staining and flow cytometry analysis
Cells were subjected to propidium iodide staining and flow cytometry analysis as previously described.6
Statistical analysis
All quantitative variables are presented as means±S.E. We compared the differences of more than two groups using one-way ANOVA and the differences of two groups using two-tailed Student's t-test (GraphPad Prism 5, La Jolla, CA, USA), and P<0.05 was considered statistically significant.
Abbreviations
- ASK1:
-
apoptosis signal-regulating kinase 1
- ATF4:
-
activating transcription factor 4
- ATM:
-
ataxia-telangiectasia mutated
- DDR:
-
DNA damage response
- DMSO:
-
dimethyl sulphoxide
- DNA-PK:
-
DNA-dependent protein kinase
- dsRNA:
-
double-stranded RNA
- eIF2α:
-
eukaryotic initiation factor 2 subunit-α
- eIF2B:
-
eukaryotic initiation factor 2B
- ER:
-
endoplasmic reticulum
- ERK:
-
extracellular signal-regulated kinase
- FACS:
-
fluorescence-activated cell sorting
- GCN2:
-
general control nonderepressible 2
- H2AX:
-
histone 2AX
- JNK:
-
c-jun N-terminal kinase
- KD:
-
kinase domain
- MAPK:
-
mitogen-activated protein kinase
- MEF:
-
mouse embryonic fibroblast
- NF-κB:
-
nuclear factor of κ-light polypeptide gene enhancer in B cells
- PERK:
-
PKR-like endoplasmic reticulum-resident kinase
- PI:
-
propidium iodide
- PI3K:
-
phosphatidylinositol 3 kinase
- PKR:
-
double-stranded RNA-dependent protein kinase
References
Holcik M, Sonenberg N . Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6: 318–327.
Wek RC, Jiang HY, Anthony TG . Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 2006; 34: 7–11.
Kazemi S, Mounir Z, Baltzis D, Raven JF, Wang S, Krishnamoorthy JL et al. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol Biol Cell 2007; 18: 3635–3644.
Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 2004; 24: 10161–10168.
Donze O, Deng J, Curran J, Sladek R, Picard D, Sonenberg N . The protein kinase PKR: a molecular clock that sequentially activates survival and death programs. EMBO J 2004; 23: 564–571.
Kazemi S, Papadopoulou S, Li S, Su Q, Wang S, Yoshimura A et al. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol Cell Biol 2004; 24: 3415–3429.
Krishnamoorthy J, Mounir Z, Raven JF, Koromilas AE . The eIF2alpha kinases inhibit vesicular stomatitis virus replication independently of eIF2alpha phosphorylation. Cell Cycle 2008; 7: 2346–2351.
Raven JF, Koromilas AE . PERK and PKR: old kinases learn new tricks. Cell Cycle 2008; 7: 1146–1150.
Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 2002; 12: 1279–1286.
Wu S, Hu Y, Wang JL, Chatterjee M, Shi Y, Kaufman RJ . Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J Biol Chem 2002; 277: 18077–18083.
von HU, Pataer A, Raju U, Bocangel D, Vorburger SA, Liu Y et al. The double-stranded RNA-activated protein kinase mediates radiation resistance in mouse embryo fibroblasts through nuclear factor kappaB and Akt activation. Clin Cancer Res 2007; 13: 6032–6039.
Bergeron J, Benlimame N, Zeng-Rong N, Xiao D, Scrivens PJ, Koromilas AE et al. Identification of the interferon-inducible double-stranded RNA-dependent protein kinase as a regulator of cellular response to bulky adducts. Cancer Res 2000; 60: 6800–6804.
Cuddihy AR, Li S, Tam NW, Wong AH, Taya Y, Abraham N et al. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol Cell Biol 1999; 19: 2475–2484.
Abraham N, Stojdl DF, Duncan PI, Methot N, Ishii T, Dube M et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 1999; 274: 5953–5962.
Baltzis D, Li S, Koromilas AE . Functional characterization of pkr gene products expressed in cells from mice with a targeted deletion of the N terminus or C terminus domain of PKR. J Biol Chem 2002; 277: 38364–38372.
Fan M, Chambers TC . Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist Updat 2001; 4: 253–267.
Silva AM, Whitmore M, Xu Z, Jiang Z, Li X, Williams BR . Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J Biol Chem 2004; 279: 37670–37676.
Gewirtz DA . A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727–741.
Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ . ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis 2003; 24: 1571–1580.
Kurz EU, Lees-Miller SP . DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst) 2004; 3: 889–900.
Cuddihy AR, Wong AH, Tam NW, Li S, Koromilas AE . The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 1999; 18: 2690–2702.
Qu L, Huang S, Baltzis D, Rivas-Estilla AM, Pluquet O, Hatzoglou M et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev 2004; 18: 261–277.
Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE . The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 2007; 282: 31675–31687.
Pluquet O, Qu L, Baltzis D, Koromilas AE . Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of Hdm2 and GSK-3beta. Mol Cell Biol 2005; 25: 9392–9405.
Barber GN . The NFAR's (nuclear factors associated with dsRNA): evolutionarily conserved members of the dsRNA binding protein family. RNA Biol 2009; 6: 35–39.
Dhanasekaran DN, Reddy EP . JNK signaling in apoptosis. Oncogene 2008; 27: 6245–6251.
Yoshida K . Regulation for nuclear targeting of the Abl tyrosine kinase in response to DNA damage. Adv Exp Med Biol 2007; 604: 155–165.
Takizawa T, Tatematsu C, Nakanishi Y . Double-stranded RNA-activated protein kinase interacts with apoptosis signal-regulating kinase 1. Implications for apoptosis signaling pathways. Eur J Biochem 2002; 269: 6126–6132.
Takeda K, Noguchi T, Naguro I, Ichijo H . Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol 2008; 48: 199–225.
Haines GK, Cajulis R, Hayden R, Duda R, Talamonti M, Radosevich JA . Expression of the double-stranded RNA-dependent protein kinase (p68) in human breast tissues. Tumour Biol 1996; 17: 5–12.
Kim SH, Forman AP, Mathews MB, Gunnery S . Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene 2000; 19: 3086–3094.
Proud CG . Regulation of eukaryotic initiation factor eIF2B. Prog Mol Subcell Biol 2001; 26: 95–114.
Balachandran S, Barber GN . Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 2004; 5: 51–65.
Ruvolo VR, Kurinna SM, Karanjeet KB, Schuster TF, Martelli AM, McCubrey JA et al. PKR regulates B56(alpha)-mediated BCL2 phosphatase activity in acute lymphoblastic leukemia-derived REH cells. J Biol Chem 2008; 283: 35474–35485.
Pataer A, Swisher SG, Roth JA, Logothetis CJ, Corn P . Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biol Ther 2009; 8: 245–252.
Harding HP, Zhang Y, Ron D . Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397: 271–274.
Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 2005; 1: 273–277.
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 2001; 7: 1165–1176.
Cheong JW, Chong SY, Kim JY, Eom JI, Jeung HK, Maeng HY et al. Induction of apoptosis by apicidin, a histone deacetylase inhibitor, via the activation of mitochondria-dependent caspase cascades in human Bcr-Abl-positive leukemia cells. Clin Cancer Res 2003; 9: 5018–5027.
Li S, Koromilas AE . Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J Biol Chem 2001; 276: 13881–13890.
Acknowledgements
We thank the Koromilas lab members for helpful comments: JE Durbin for PKR+/+ and PKR−/− MEFs; D Ron for PERK+/+ and PERK−/− MEFs, GCN2+/+ and GCN2−/− MEFs and R Kaufman for eIF2αS/S and eIF2αA/A MEFs. PP is the recipient of the Montreal Centre for Experimental Therapeutics in Cancer (MCETC) post-doctoral award. AIP is the recipient of the Canadian Institutes of Health Research (CIHR) Frederick Banting Charles Best Canadian Graduate Scholarship. HM is the recipient of the Fonds de la Reserche en Sante Quebec (FRSQ) Master's Training Award. This work was supported by funds form the Canadian Cancer Society Research Institute (CCSRI; Grant no. 017285) to AEK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Peidis, P., Papadakis, A., Muaddi, H. et al. Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death. Cell Death Differ 18, 145–154 (2011). https://doi.org/10.1038/cdd.2010.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2010.76
Keywords
This article is cited by
-
Modulating the integrated stress response to slow aging and ameliorate age-related pathology
Nature Aging (2021)
-
Protein kinase RNA-activated controls mitotic progression and determines paclitaxel chemosensitivity through B-cell lymphoma 2 in ovarian cancer
Oncogene (2021)
-
An integrated stress response via PKR suppresses HER2+ cancers and improves trastuzumab therapy
Nature Communications (2019)
-
NDRG2 contributes to cisplatin sensitivity through modulation of BAK-to-Mcl-1 ratio
Cell Death & Disease (2018)
-
Sall2 is required for proapoptotic Noxa expression and genotoxic stress-induced apoptosis by doxorubicin
Cell Death & Disease (2015)