Abstract
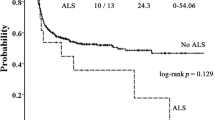
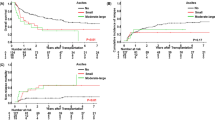
The objective of this study was to assess the incidence, risk factors, outcome and impact on OS of pericardial effusion (PEF) in a cohort of 156 pediatric SCT recipients. The mean age was 8.15±6.25 years. In all, 74% of the patients had malignant disease and 35% of the patients received autologous stem cell grafts. Twenty-three subjects developed effusion at 2.75±3.54 months after SCT. The overall probability of developing a PEF after SCT was 16.9%. In the multivariate analysis of risk factors associated with time to PEF, increased age, allogeneic risk status and conditioning type, were all significant factors. In a multivariate analysis of time to death, PEF, CMV status and risk status were all independent risk factors. PEF, however, had the highest HR of 3.334. Of the 23 patients with PEF, 19 died (82.6%); however, none died as a direct result of pericardial tamponade. In summary, our results suggest that PEF is a significant risk factor for post transplant mortality. These results suggest a need for more frequent evaluation and monitoring for development of PEF. Studies are needed to determine the etiology of, and new therapeutic strategies for, PEF in the post-SCT population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cairo M, Heslop H . Pediatric blood and marrow transplantation: state of the science. Bone Marrow Transplant 2008; 41: 97.
Murdych T, Weisdorf DJ . Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant 2001; 28: 283–287.
Einsele H, Ehninger G, Vallbracht A, Kompf J, Schmidt H, Kandolf R et al. Isolated pericardial relapse following allogeneic bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant 1989; 4: 323–325.
Krishnan GS, Chaudhary V, Al-Janadi A, Ramanarayanan J, D’Silva KJ . BCNU toxicity presenting with a large pericardial and pleural effusion. Ann Transplant 2008; 13: 44–47.
Rhodes M, Lautz T, Kavanaugh-Mchugh A, Manes B, Calder C, Koyama T et al. Pericardial effusion and cardiac tamponade in pediatric stem cell transplant recipients. Bone Marrow Transplant 2005; 36: 139–144.
Saito Y, Matsushima T, Doki N, Tsumita Y, Takizawa M, Yokohama A et al. Pericardial graft vs. host disease in a patient with myelodysplastic syndrome following peripheral blood stem cell transplantation. Eur J Haematol 2005; 75: 65–67.
Seber A, Khan SP, Kersey JH . Unexplained effusions: association with allogeneic bone marrow transplantation and acute or chronic graft-versus-host disease. Bone Marrow Transplant 1996; 17: 207–211.
Silberstein L, Davies A, Kelsey S, Foran J, Murrell C, D’Cruz D et al. Myositis, polyserositis with a large pericardial effusion and constrictive pericarditis as manifestations of chronic graft-versus-host disease after non-myeloablative peripheral stem cell transplantation and subsequent donor lymphocyte infusion. Bone Marrow Transplant 2001; 27: 231–233.
Toren A, Nagler A . Massive pericardial effusion complicating the course of chronic graft-versus-host disease (cGVHD) in a child with acute lymphoblastic leukemia following allogeneic bone marrow transplantation. Bone Marrow Transplant 1997; 20: 805–807.
Veys PA, McAvinchey R, Rothman MT, Mair GH, Newland AC . Pericardial effusion following conditioning for bone marrow transplantation in acute leukaemia. Bone Marrow Transplant 1987; 2: 213–216.
Angelucci E, Mariotti E, Lucarelli G, Baronciani D, Cesaroni P, Durazzi SM et al. Sudden cardiac tamponade after chemotherapy for marrow transplantation in thalassaemia. Lancet 1992; 339: 287–289.
Cereda M, Trocino G, Pogliani EM, Schiavina R . A case of cardiac localization of graft-versus-host disease after allogenic bone marrow transplantation. Ital Heart J 2003; 4: 60–63.
Kondo M, Kojima S, Horibe K, Kato K, Matsuyama T . Hemolytic uremic syndrome after allogeneic or autologous hematopoietic stem cell transplantation for childhood malignancies. Bone Marrow Transplant 1998; 21: 281–286.
Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant 2004; 10: 246–258.
Yamashiro DJ, Lee A, Bhatia M, Glade-Bender J, Qualter E, Militano O et al. Feasibility of autologous stem cell transplant followed by reduced intensity allogeneic stem cell transplantation for high risk neuroblastoma: a single institution pilot study. Biol Blood Marrow Transplant 2007; 13: 68–69 (abstract).
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Roman E, Osunkwo I, Militano O, Cooney E, van de Ven C, Cairo MS . Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer 2008; 50: 325–330.
Shereck EB, Cooney E, van de Ven C, Della-Lotta P, Cairo MS . A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer 2007; 49: 306–312.
Waxman IM, Militano O, Baldinger L, Roman E, Qualter E, Morris E et al. Sequential administration of sargramostim and filgrastim in pediatric allogeneic stem cell transplantation recipients undergoing myeloablative conditioning. Pediatr Transplant 2009; 13: 464–474.
Anasetti C . What are the most important donor and recipient factors affecting the outcome of related and unrelated allogeneic transplantation? Best Pract Res Clin Haematol 2008; 21: 691–697.
Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 2007; 109: 3658–3666.
Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008; 111: 1827–1833.
Doney K, Hagglund H, Leisenring W, Chauncey T, Appelbaum FR, Storb R . Predictive factors for outcome of allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2003; 9: 472–481.
Satwani P, Morris E, Bradley MB, Bhatia M, van de Ven C, Cairo MS . Reduced intensity and non-myeloablative allogeneic stem cell transplantation in children and adolescents with malignant and non-malignant diseases. Pediatr Blood Cancer 2008; 50: 1–8.
Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J . Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant 2003; 9: 543–558.
Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol 2008; 26: 5735–5741.
Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS . In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood 2009; 113: 1365–1374.
Chung HT, Hsieh TC, Yu MC, Chang YS, Lo WC, Jaing TH . Staphylococcus aureus pericardial abscess in a child with beta-thalassemia major following double-unit unrelated cord blood transplantation. Pediatr Hematol Oncol 2007; 24: 275–279.
Schaumann R, Ponisch W, Helbig JH, Hegenbart U, Ackermann G, Hofmann J et al. Pericarditis after allogeneic peripheral blood stem cell transplantation caused by Legionella pneumophila (non-serogroup 1). Infection 2001; 29: 51–53.
Acknowledgements
This work was supported in part by grants from the Pediatric Cancer Research Foundation, Brittany Baron Fund, Sonia Scaramella Fund, Bevanmar Foundation, Paul Luisi Foundation, Dreaming for Discovery and Cure Fund, and the Marisa Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented in part at the American Society of Pediatric Hematology/Oncology, Toronto, Canada, May 2007 and American Society of Blood and Marrow Transplantation, San Diego, CA, February 2008.
Rights and permissions
About this article
Cite this article
Neier, M., Jin, Z., Kleinman, C. et al. Pericardial effusion post-SCT in pediatric recipients with signs and/or symptoms of cardiac disease. Bone Marrow Transplant 46, 529–538 (2011). https://doi.org/10.1038/bmt.2010.149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.149
Keywords
This article is cited by
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)
-
Anatomic Approach and Outcomes in Children Undergoing Percutaneous Pericardiocentesis
Pediatric Cardiology (2021)
-
Predictors and Outcome of Pericardial Effusion After Hematopoietic Stem Cell Transplantation in Children
Pediatric Cardiology (2018)
-
Pericardial effusion requiring surgical intervention after stem cell transplantation: a case series
Bone Marrow Transplantation (2017)
-
The injured heart: early cardiac effects of hematopoietic stem cell transplantation in children and young adults
Bone Marrow Transplantation (2017)