Abstract
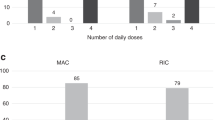
We studied the pharmacokinetic (PK) profile of single daily dose i.v. BU in children who underwent reduced-intensity conditioning (RIC) transplantation. A cohort of 19 patients ⩽4 years of age (group 1) and 33 patients >4 years (group 2) was studied. Patients received a BU test dose for PK studies, followed by two treatment doses adjusted to target an area under the curve (AUC) of 4000 μM min per day. Patients in group 1 attained a lower AUC as compared to group 2 (3568 vs 4035 μM min). In group 1, 67% patients and in group 2, 84% patients achieved AUC within the targeted range. Stable donor chimerism was achieved in 56% patients in group 1 and 79% in group 2. Eight patients required a second transplantation because of graft failure. Because of the concern that a low AUC adversely affected outcomes, a second cohort of 23 patients followed a modified protocol with a targeted AUC of 5000 μM min. A higher AUC was attained (4825 μM min). Stable donor chimerism was achieved in 91% of patients. Our results show that RIC regimens using two single daily doses of i.v. BU are effective in children, but a targeted AUC of 5000 μM min is recommended.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hassan M . The role of busulfan in bone marrow transplantation. Med Oncol 1999; 16: 166–176.
Hoy SM, Lyseng-Williamson KA . Intravenous busulfan in the conditioning treatment of pediatric patients prior to hematopoietic stem cell transplantation. Paediatr Drugs 2007; 9: 271–278.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Dupuis LL, Najdova M, Saunders EF . Retrospective appraisal of busulfan dose adjustment in children. Bone Marrow Transplant 2000; 26: 1143–1147.
Copelan EA, Bechtel TP, Avalos BR, Elder PJ, Ezzone SA, Scholl MD et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complications following marrow transplantation. Bone Marrow Transplant 2001; 27: 1121–1124.
Grochow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20: 18–25.
Hassan M, Ljungman P, Bolme P, Ringden O, Syruckova Z, Bekassy A et al. Busulfan bioavailability. Blood 1994; 84: 2144–2150.
Vassal G, Koscielny S, Challine D, Valteau-Couanet D, Boland I, Deroussent A et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–253.
Bostrom B, Enockson K, Johnson A, Bruns A, Blazar B . Plasma pharmacokinetics of high-dose oral busulfan in children and adults undergoing bone marrow transplantation. Pediatr Transplant 2003; 7 (Suppl 3): 12–18.
Oechtering D, Schiltmeyer B, Hempel G, Schwab M, Wurthwein G, Murdter T et al. Toxicity and pharmacokinetics of i.v. busulfan in children before stem cell transplantation. Anticancer Drugs 2005; 16: 337–344.
Tran H, Petropoulos D, Worth L, Mullen CA, Madden T, Andersson B et al. Pharmacokinetics and individualized dose adjustment of intravenous busulfan in children with advanced hematologic malignancies undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 805–812.
Grochow LB . Parenteral busulfan: is therapeutic monitoring still warranted? Biol Blood Marrow Transplant 2002; 8: 465–467.
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
Kletzel M, Jacobsohn D, Duerst R . Pharmacokinetics of a test dose of intravenous busulfan guide dose modifications to achieve an optimal area under the curve of a single daily dose of intravenous busulfan in children undergoing a reduced-intensity conditioning regimen with hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12: 472–479.
Zwaveling J, den Hartigh J, Lankester AC, Guchelaar HJ, Egeler RM, Bredius RG . Once-daily intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Anticancer Drugs 2006; 17: 1099–1105.
Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant 2007; 13: 56–64.
Russell JA, Duan Q, Chaudhry MA, Savoie ML, Balogh A, Turner AR et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant 2008; 14: 888–895.
Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant 2008; 14: 672–684.
Grochow LB, Krivit W, Whitley CB, Blazar B . Busulfan disposition in children. Blood 1990; 75: 1723–1727.
Hassan M, Oberg G, Bekassy AN, Aschan J, Ehrsson H, Ljungman P et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol 1991; 28: 130–134.
Regazzi MB, Locatelli F, Buggia I, Bonetti F, Zecca M, Pregnolato M et al. Disposition of high-dose busulfan in pediatric patients undergoing bone marrow transplantation. Clin Pharmacol Ther 1993; 54: 45–52.
Hassan M, Fasth A, Gerritsen B, Haraldsson A, Syruckova Z, van den Berg H et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant 1996; 18: 843–850.
Gibbs JP, Murray G, Risler L, Chien JY, Dev R, Slattery JT . Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res 1997; 57: 5509–5516.
Vassal G, Fischer A, Challine D, Boland I, Ledheist F, Lemerle S et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood 1993; 82: 1030–1034.
Pawlowska AB, Blazar BR, Angelucci E, Baronciani D, Shu XO, Bostrom B . Relationship of plasma pharmacokinetics of high-dose oral busulfan to the outcome of allogeneic bone marrow transplantation in children with thalassemia. Bone Marrow Transplant 1997; 20: 915–920.
Bertholle-Bonnet V, Bleyzac N, Galambrun C, Mialou V, Bertrand Y, Souillet G et al. Influence of underlying disease on busulfan disposition in pediatric bone marrow transplant recipients: a nonparametric population pharmacokinetic study. Ther Drug Monit 2007; 29: 177–184.
Dalle JH, Wall D, Theoret Y, Duval M, Shaw L, Larocque D et al. Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results. Bone Marrow Transplant 2003; 32: 647–651.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Lindley C, Shea T, McCune J, Shord S, Decker J, Harvey D et al. Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy. Anticancer Drugs 2004; 15: 453–459.
Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C . I.v. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant 2004; 33: 979–987.
Booth BP, Rahman A, Dagher R, Griebel D, Lennon S, Fuller D et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol 2007; 47: 101–111.
Vassal G, Michel G, Esperou H, Gentet JC, Valteau-Couanet D, Doz F et al. Prospective validation of a novel iv busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol 2008; 61: 113–123.
Jacobsohn DA, Duerst R, Tse W, Kletzel M . Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet 2004; 364: 156–162.
Duerst RE, Jacobsohn D, Tse WT, Kletzel M . Efficacy of reduced intensity conditioning with Flu-Bu-ATG and allogeneic hematopoietic stem cell transplantation for pediatric ALL. Blood 2004; 104 (Suppl 1): 636a (abstract).
Schneiderman J, Duerst R, Tse W, Thormann K, Kletzel M, Jacobsohn D . Reduced intensity conditioning and outpatient hematopoietic stem cell transplantation using photophoresis, fludarabine and targeted dose busulfan in children. Biol Blood Marrow Transplant 2007; 13: 65–66 (abstract).
Wall D, Chan KW, Neider M, Feingold J, Hayashi R, Yeager A et al. Phase II trial of intravenous busulfan with cyclophosphamide in pediatric allogeneic hematopoietic stem cell transplantation: pharmacokinetics, toxicity and efficiency. Blood 2000; 96 (Suppl 1): 480a (abstract).
Wang LJ, Chou P, Gonzalez-Ryan L, Huang W, Haut PR, Kletzel M . Evaluation of mixed hematopoietic chimerism in pediatric patients with leukemia after allogeneic stem cell transplantation by quantitative PCR analysis of variable number of tandem repeat and testis determination gene. Bone Marrow Transplant 2002; 29: 51–56.
Schechter T, Finkelstein Y, Doyle J, Verjee Z, Moretti M, Koren G et al. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 307–314.
Nath CE, Earl JW, Pati N, Stephen K, Shaw PJ . Variability in the pharmacokinetics of intravenous busulphan given as a single daily dose to paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 2008; 66: 50–59.
Chattergoon DS, Saunders EF, Klein J, Calderwood S, Doyle J, Freedman MH et al. An improved limited sampling method for individualised busulphan dosing in bone marrow transplantation in children. Bone Marrow Transplant 1997; 20: 347–354.
Tran HT, Madden T, Petropoulos D, Worth LL, Felix EA, Sprigg-Saenz HA et al. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Bone Marrow Transplant 2000; 26: 463–470.
Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant 2001; 28: 743–751.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Hassan M, Nilsson C, Hassan Z, Gungor T, Aschan J, Winiarski J et al. A phase II trial of liposomal busulphan as an intravenous myeloablative agent prior to stem cell transplantation: 500 mg/m(2) as a optimal total dose for conditioning. Bone Marrow Transplant 2002; 30: 833–841.
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Bartelink IH, Bredius RG, Ververs TT, Raphael MF, van Kesteren C, Bierings M et al. Once-daily intravenous busulfan with therapeutic drug monitoring compared to conventional oral busulfan improves survival and engraftment in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14: 88–98.
Acknowledgements
We thank Kimberly Thormann, Jessica Guarino, Alexis Baby, Kelly Coyne, Mary Stoelinga, Theresa Morrison and the nurses of the Ambulatory Stem Cell Unit and the Oncology Unit for skillful management of patients, and Marvin M Goldenberg for editorial review.
This work was supported by a restricted grant from ESP Pharmaceuticals Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
None of the authors have a financial interest in ESP Pharmaceuticals Inc., whose product IV Busulfex was studied in this work.
Rights and permissions
About this article
Cite this article
Tse, W., Duerst, R., Schneiderman, J. et al. Age-dependent pharmacokinetic profile of single daily dose i.v. busulfan in children undergoing reduced-intensity conditioning stem cell transplant. Bone Marrow Transplant 44, 145–156 (2009). https://doi.org/10.1038/bmt.2008.437
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.437
Keywords
This article is cited by
-
Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients
Clinical Pharmacokinetics (2021)
-
The pharmacokinetics and pharmacodynamics of busulfan when combined with melphalan as conditioning in adult autologous stem cell transplant recipients
Annals of Hematology (2018)
-
Optimizing drug therapy in pediatric SCT: Focus on pharmacokinetics
Bone Marrow Transplantation (2015)