Abstract
Background:
Whether females have better survival than males in nasopharyngeal carcinoma is barely acknowledged and the exact explanations remain unknown.
Methods:
Overall, 5929 patients receiving treatment between January 2005 and December 2010 were separately stratified by stage into early and advanced stage groups, and by age into premenopausal (⩽45 years), menopausal (46–54 years) and postmenopausal (⩾55 years) groups. Matched males and females in each group were identified using the propensity score matching method. Differences in disease-free survival (DSS), overall survival (OS), distant metastasis-free survival (DMFS) and locoregional relapse-free survival (LRFS) were estimated by the Kaplan–Meier method and Cox regression model.
Results:
Overall, 398, 923, 744, 319 and 313 pairs of males and females were matched in early stage, advanced stage, premenopausal, menopausal and postmenopausal group, respectively. Females showed significant advantage over males across all end points in both early and advanced stage groups (P⩽0.042). However, this advantage persisted at premenopausal age (P⩽0.042), declined during menopause (DMFS, P=0.021; DSS, P=0.100; OS, P=0.693; LRFS, P=0.330) and totally disappeared at postmenopausal age (P⩾0.344).
Conclusions:
Sex significantly affects NPC survival, with a definite female advantage regardless of tumour stage. Intrinsic biologic traits appear to be the exact explanation according to the declining magnitude of sex effect with age.
Similar content being viewed by others
Main
Nasopharyngeal carcinoma (NPC) is a malignancy with unique geographic distribution. It is rare in Europe and the United States, with an incidence of 0.5–2 per 100 000 (Ferlay et al, 2004). By contrast, NPC is endemic in Southern China (Cao et al, 2011) and Hong Kong (Chang and Adami, 2006) where the incidence can be as high as 20–30 per 100 000. There is a common feature of the incidence rates almost in all populations—the male predominance, with a male-to-female ratio of 2–3 : 1 (Ferlay et al, 2004).
With regard to the prognostic effect of sex on the treatment outcomes of patients with established NPC, significant female advantage in survival was found in a randomised controlled trial about chemotherapy (Lee et al, 2005), but null results were reported in the other three trials (Chen et al, 2011, 2012; Fountzilas et al, 2012). Although female NPC patients were found to have higher survival rates than male counterparts in a retrospective comparison (Xiao et al, 2013), the causes remain confused. It was previously assumed that behavioural differences across sex, especially diagnostic delays, might contribute to the observed sex differences in survival of caner of oesophagus (Bohanes et al, 2012) and melanoma (Joosse et al, 2013). According to the population-based evidence of age-dependent sex ratio in the incidence of NPC with an inflection at menopause ages and a delay of developing NPC in females before menopause (Xie et al, 2013), we proposed another hypothesis of the intrinsic biologic sex differences, mainly about the protective effect of oestrogen. If the first assumption in other cancers can be applied to NPC, then it is of great necessity to fully balance the interactions of sex and other behavioural prognoses, especially tumour stage. If the second hypothesis holds, then it is important to detect the survival advantage of female sex in all types of disease progression (e.g., both locoregional relapse and distant metastasis) on one side, and no survival superiority of female sex among postmenopausal patients on the other.
To well balance the influence of covariates, we compared the survival outcomes of male and female NPC patients using the propensity score matching method (Baser, 2006; Austin, 2009) and multivariate analysis. To clarify the exact explanations, the influence of behavioural factors and intrinsic biologic trait was further analysed by assessing the magnitude of the prognostic effect of sex in different groups.
Patients and methods
Patients
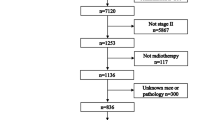
This retrospective study was approved by the Institutional Review Board at Sun Yat-sen University Cancer Center, and individual informed consent was waived given the anonymous analysis of routine data. Between January 2005 and December 2010, 5929 newly diagnosed, biopsy-proven, non-metastatic and hospitalised NPC patients who were at the age of 20 or above were entered into this study. All patients had complete pretreatment evaluation including patient history, physical examination, haematology and biochemistry profiles, fiberoptic nasopharyngoscopy with biopsy, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography and Technetium-99 m-methylene diphosphonate (Tc-99-MDP) whole-body bone scan. Patients were restaged according to the seventh edition of the International Union against Cancer/American Joint Committee on Cancer staging system for NPC (Edge et al, 2010).
Treatment
All patients were treated by definitive intensity-modulated radiotherapy (IMRT) or conventional radiotherapy (CRT) with or without chemotherapy. Further details of the radiation techniques used at our centre have been described previously (Lai et al, 2011). Institutional guidelines recommended no chemotherapy for patients in early stage, and induction, concurrent and adjuvant chemotherapy or combined treatment for those in locoregionally advanced stage. Induction or adjuvant chemotherapy consisted of cisplatin with 5-fluorouracil, cisplatin with taxane or triplet of cisplatin and 5-fluorouracil plus taxane every 3 weeks for 2–3 cycles. Concurrent chemotherapy consisted of cisplatin given on weeks 1, 4 and 7 of radiotherapy or cisplatin given weekly. Deviation from the institutional guidelines was result from organ dysfunction, treatment intolerance and/or patient refusal.
Follow-up
Patients were examined every 3–6 months during the first 3 years, with follow-up examinations every 6–12 months thereafter until death. During this period, patients were assessed by history and physical examination and a series of conventional examination equipment at each follow-up visit, to detect the possible relapse or distant metastasis. Local relapses were confirmed by biopsy, MRI scan, or both. Regional relapses were diagnosed by clinical examination and an MRI scan of the neck and, in doubtful cases, by fine needle aspiration of the lymph nodes. Distant metastases were diagnosed by clinical symptoms, physical examinations and imaging methods including chest radiography, bones scan, MRI and abdominal sonography. Patients without recent examination tests in the medical records were followed up by telephone call.
Statistical analysis
We selected male patients who were matched with the female counterparts using propensity score matching. This is a method for creating similar case (female) and control (male) sets from an existing data set on the presumed covariates, to reduce possible biases to a minimum in a retrospective analysis (D'Agostino, 1998). Propensity scores were computed by logistic regression for each patient based on the following covariates, including age, smoking, drinking, histology, titres of immunoglobulin A against viral capsid antigen (VCA-IgA) and early antigen (EA-IgA), body mass index (BMI), T-stage, N-stage, clinical stage, radiation techniques and chemotherapy regimens. Female and male patients were then matched without replacement at the ratio of 1 : 1 on those scores, rather than the individual covariates.
To test the individual hypothesis, 5929 patients were independently stratified by clinical stage and age into five groups: early stage (stage I+II) group and advanced stage (stage III+IVa-b) group according to clinical stage; premenopausal age (⩽45 years) group, menopausal age (46–54 years) group and postmenopausal age (⩾55 years) group according to age. Propensity score matching was utilised to identify the matched female and male patients in each group for subsequent analysis.
Covariates balance between female and male was examined by t test (continuous variable), χ2 test or Fisher’s exact test (categorical variable) as appropriate. Disease-specific survival (DSS), overall survival (OS), distant metastasis-free survival (DMFS) and locoregional relapse-free survival (LRFS) were estimated with the Kaplan–Meier method (Kaplan and Meier, 1958). Crude and adjusted hazard ratios with 95% confidence intervals for sex (with male sex as reference) were calculated using Cox regression analysis or Cox regression model with time-dependent covariates if the proportional hazards assumption did not hold (Cox, 1972). DSS, OS, LRFS and DMFS were defined as the time from treatment to death resulting from NPC or treatment complications, to death from any cause, to the first locoregional relapse and to the first distant metastasis, respectively.
All statistical analyses were performed using IBM SPSS Statistics version 22.0 (http://www-01.ibm.com/software/analytics/spss/downloads.html). Two-sided P-values<0.05 were considered to be significant.
Results
Patients
From the original 473 female vs 1274 male patients with stage I/II and 1029 female vs 3153 male patients with stage III/IVa-b (Supplementary Table 1), 398 and 923 pairs were respectively selected by propensity score matching (Table 1). The median follow-up time for the included patients in both groups was 61.78 months (4.37–109.23 months) and 53.65 months (3.50–110.67 months), respectively.
From the original 821 female vs 2194 male patients at premenopausal age, 359 female vs 1181 male patients at menopausal age and 322 female vs 1052 male patients at postmenopausal age (Supplementary Table 2), 744, 319 and 313 pairs were respectively selected by propensity score matching (Table 2). The median follow-up time for the included patients in the three groups was 58.27 months (3.50–110.67 months), 54.67 months (3.73–109.23 months) and 50.97 months (3.67–111.07 months), respectively.
The baseline characteristics of all patients before matching (Supplementary Tables 1 and 2) and the excluded patients by matching (Supplementary Tables 3 and 4) were statistically different across sex. However, the included males and females after matching in each group had similar mean of age and BMI, smoking status, drinking status, histology, titres of VCA-IgA and EA-IgA, T-stage, N-stage, clinical stage, radiation techniques and chemotherapy regimens (Tables 1 and 2).
Survival outcomes
Compared with male patients, female counterparts showed significant advantage across all end points in both early stage (DSS rates at 5 years 97.1% vs 91.7%, P=0.002; OS rates at 5 years 97.1% vs 91.7%, P=0.003; DMFS rates at 5 years 97.9% vs 93.4%, P=0.006; and LRFS rates at 5 years 94.8% vs 92.0%, P=0.017; Figure 1A–D) and advanced stage groups (DSS rates at 5 years 86.2% vs 80.7%, P=0.014; OS rates at 5 years 85.8% vs 80.6%, P=0.021; DMFS rates at 5 years 85.5% vs 80.4%, P=0.006; and LRFS rates at 5 years 91.5% vs 87.5%, P=0.042; Figure 1E–H).
However, this female survival advantage across all end points was limited at premenopausal age (⩽45 years) (DSS rates at 5 years 91.5% vs 87.1%, P=0.015; OS rates at 5 years 91.3% vs 87.3%, P=0.023; DMFS rates at 5 years 89.8% vs 85.4%, P=0.028; and LRFS rates at 5 years 91.6% vs 88.4%, P=0.042; Figure 2A–D). There were no significant differences in DSS (rates at 5 years 91.0% vs 87.0%, P=0.100), OS (rates at 5 years 90.7% vs 86.8%, P=0.693) or LRFS (rates at 5 years 93.4% vs 91.7%, P=0.330) between female and male patients at menopausal age (46–54 years), except DMFS (rates at 5 years 91.9% vs 86.0%, P=0.021). (Figure 2E–H) Furthermore, the female survival advantage totally vanished when patients reached the postmenopausal age (⩾55 years) (DSS rates at 5 years 78.9% vs 78.4%, P=0.525; OS rates at 5 years 78.9% vs 78.0%, P=0.518; DMFS rates at 5 years 86.8% vs 85.6%, P=0.344; and LRFS rates at 5 years 88.4% vs 89.3%, P=0.509; Figure 2I–L).
Multivariate analysis
Accounting for age (continuous), smoking, drinking, histology, titres of VCA-IgA (<80/80–320/⩾320) and EA-IgA (<10/10–40/⩾40), BMI (continuous), T-stage, N-stage, clinical stage, radiation techniques and chemotherapy regimens in multivariate analysis, the significant female advantage persisted for DSS, OS, DMFS and LRFS, regardless of clinical stage (Table 3).
With adjustment for the same covariates, female sex was an independent, significant protective predictor of DSS, OS, DMFS and LRFS for patients at premenopausal age, along with DMFS for patients at menopausal age. However, sex was not significantly associated with the DSS, OS or LRFS of patients at menopausal age, or any of the end points of patients at postmenopausal age (Table 4).
Discussion
The most appealing result of this study is the convincing prognostic advantage in DSS, OS, DMFS and LRFS from female sex for patients with nasopharyngeal carcinoma using the propensity score matching analysis. Currently, this propensity score matching analysis, along with multivariate analysis, provides the fairest comparison of matched male and female patients to evaluate the sex effect. This protective effect of female sex is fairly consistent with that reported in the literature for NPC (Lee et al, 2005; Xiao et al, 2013) and other cancers (Hidaka et al, 2007; McGovern et al, 2009; Bohanes et al, 2012; Cheung et al, 2013; Joosse et al, 2013).
The remarkable sex differences in survival were used to be presumably explained by sex differences in lifestyle behaviour and diagnostic delays in other cancers (Bohanes et al, 2012; Joosse et al, 2013). However, in this propensity-matched study, several behavioural factors (e.g., smoking and drinking status and BMI) and multiple indicators related to diagnostic delays (e.g., T-stage, N-stage, clinical stage, titre of VCA-IgA and EA-IgA) were well balanced, and even taking these confounders into account caused little shift from crude to adjusted hazard ratios and failed to overturn the significant sex effect. More importantly, sex remained the independent prognostic value across all end points (DSS, OS, DMFS and LRFS) in locoregionally advanced nasopharyngeal carcinoma, as demonstrated in patients with early stage, although the relative female advantage declined from the 51–67% advantage in early stage to a nearly 30% advantage in advanced stage (Table 3). Therefore, our findings indicate that similar to oesophageal cancer (Bohanes et al, 2012) and melanoma (Joosse et al, 2013), common lifestyle behaviours and diagnostic delays cannot fully explained the survival differences of male and female NPC patients, and the underlying biologic traits of female sex may have a pivotal role in a much more profound way.
The hormonal differences, especially oestrogen and oestrogen receptor (ER), are very representative of the biologic traits. The levels of oestrogen and ER in the female are known to differ before and after menopause; so there is an oestrogen and ER hypothesis that in postmenopausal women, the survival advantage from oestrogen against men should decline or even completely vanish. Since age is commonly considered as a surrogate for menopause, and only 5% of women enter menopause after age 55 years (McKinlay et al, 1992), the survival differences of male and female patients were examined in three age groups. We found that the sex differences existed across all end points in premenopausal age (⩽45 years), restricted to DMFS in menopausal age (46–54 years), and totally disappeared after menopause (⩾55 years). Therefore, this finding highly supports the oestrogen and ER hypothesis. Actually, this oestrogen-related sex disparity had already been displayed in the incidence of developing nasopharyngeal carcinoma (Xie et al, 2013). Unfortunately, little is known about the association of female hormone and survival or the underlying mechanism. This is likely to be the functional result of genetic variants, for example, the VEGF-2578 CC genotype, which was associated with tumour progression and frequently involved with the male patients as indicated by Nasr et al (2008). Additionally, it was reported that inhibition of ER-α with a repressor (NAG7) could promote nasopharyngeal carcinoma invasion via upregulation of JNK2/AP-1/MMP1 pathways (Huang et al, 2009).
Apart from the hormonal differences, another way in which the intrinsic biologic traits of sex directly exert is the response rate and probability of side effects from treatment, especially the chemotherapy. Sex-biased expression levels of metabolic enzymes and transporters in liver and kidney lead to different pharmacokinetics for most common anti-cancer drugs. In women, half-life is often longer, which exactly results in a better response rate of cisplatin in female NPC without increasing toxicity (Schmetzer and Florcken, 2012). Finally, other literature-mentioned plausible explanations for the female advantage include the differences in immune homeostasis (Bouman et al, 2005) and body iron stores (Mascitelli and Goldstein, 2013). Further researches are warranted to confirm or exclude any of these hypothetical biologic explanations.
The major strength of this study lies in the investigation of sex effect in nasopharyngeal carcinoma using propensity score matching and multivariate analysis. This directly addressed the limitations of divergent confounders, treatment heterogeneity and selection bias associated with the retrospective assessment of observational data (Austin, 2009). Additional strength is that the common hypotheses to explain the sex differences were tested, for the first time, in separate groups of matched male and female nasopharyngeal carcinoma patients.
Anyway, it was a limitation that the presented data were derived from a single institution in endemic area with expertise in diagnosing and treating this disease. Moreover, since data on DNA copy number of the Epstein-Barr virus were missing in most of cases, VCA-IgA and EA-IgA were taken as the surrogate. Finally, true anamnesis on menopausal status, data on hormonal analysis and information on hormone replacement therapy were missing in this retrospective study. However, stratified analysis by three age groups was a valuable alternative to indirectly disclose the correlation of survival differences across sex with hormone, because age is commonly considered as a surrogate for menopause. These issues would be addressed in the coming prospective study.
In conclusion, sex significantly affected the survival of nasopharyngeal carcinoma, with a definite female advantage across all end points, independent of other prognostic factors. This female survival advantage persisted in all stages of this cancer, but disappeared among postmenopausal women. It was strongly associated with the underlying biologic traits of sex, rather than the behavioural sex disparities. Sex is of great necessity to be stratified for analysis in the upcoming randomised controlled trials.
Change history
28 April 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Austin PC (2009) The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making 29 (6): 661–677.
Baser O (2006) Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health 9 (6): 377–385.
Bohanes P, Yang D, Chhibar RS, Labonte MJ, Winder T, Ning Y, Gerger A, Benhaim L, Paez D, Wakatsuki T, Loupakis F, El-Khoueiry R, Zhang W, Lenz HJ (2012) Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 30 (18): 2265–2272.
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11 (4): 411–423.
Cao SM, Simons MJ, Qian CN (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30 (2): 114–119.
Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15 (10): 1765–1777.
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J (2012) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 13 (2): 163–171.
Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, Li NW, Xiang YQ, Luo DH, Qiu F, Sun R, Deng MQ, Chen MY, Hua YJ, Guo X, Cao KJ, Hong MH, Qian CN, Mai HQ (2011) Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 103 (23): 1761–1770.
Cheung WY, Shi Q, O'Connell M, Cassidy J, Blanke CD, Kerr DJ, Meyers J, Van Cutsem E, Alberts SR, Yothers G, Sargent DJ (2013) The predictive and prognostic value of sex in early-stage colon cancer: a pooled analysis of 33,345 patients from the ACCENT database. Clin Colorectal Cancer 12 (3): 179–187.
Cox DR (1972) Regression models and life tables. J R Stat Soc B 34: 187–220.
D'Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17 (19): 2265–2281.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual 7th edn Springer: New York.
Ferlay J, Bray F, Pisani P, Parkin DM (2004) Cancer Incidence, Mortality and Prevalence Worldwide 2.0, edn IARC Press: Lyon.
Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, Zaramboukas T, Nikolaou A, Markou K, Resiga L, Dionysopoulos D, Samantas E, Athanassiou H, Misailidou D, Skarlos D, Ciuleanu T (2012) Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 23 (2): 427–435.
Hidaka H, Hotokezaka M, Nakashima S, Uchiyama S, Maehara N, Chijiiwa K (2007) Sex difference in survival of patients treated by surgical resection for esophageal cancer. World J Surg 31 (10): 1982–1987.
Huang C, Wu M, Tang Y, Li X, Ouyang J, Xiao L, Li D, Li G (2009) NAG7 promotes human nasopharyngeal carcinoma invasion through inhibition of estrogen receptor alpha and up-regulation of JNK2/AP-1/MMP1 pathways. J Cell Physiol 221 (2): 394–401.
Joosse A, Collette S, Suciu S, Nijsten T, Patel PM, Keilholz U, Eggermont AM, Coebergh JW, de Vries E (2013) Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: a pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol 31 (18): 2337–2346.
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53: 457–481.
Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J (2011) How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 80 (3): 661–668.
Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, Siu L, Sze WM, Leung TW, Sham JS, Ngan RK, Law SC, Yau TK, Au JS, O'Sullivan B, Pang ES, O SK, Au GK, Lau JT (2005) Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 23 (28): 6966–6975.
Mascitelli L, Goldstein MR (2013) Explaining sex difference in cancer risk: Might it be related to excess iron? Int J Cancer 133 (9): 2261–2262.
McGovern SL, Liao Z, Bucci MK, McAleer MF, Jeter MD, Chang JY, O'Reilly MS, Cox JD, Allen PK, Komaki R (2009) Is sex associated with the outcome of patients treated with radiation for nonsmall cell lung cancer? Cancer 115 (14): 3233–3242.
McKinlay SM, Brambilla DJ, Posner JG (1992) The normal menopause transition. Maturitas 14 (2): 103–115.
Nasr HB, Chahed K, Bouaouina N, Chouchane L (2008) Functional vascular endothelial growth factor -2578 C/A polymorphism in relation to nasopharyngeal carcinoma risk and tumor progression. Clin Chim Acta 395 (1-2): 124–129.
Schmetzer O, Flörcken A (2012) Sex differences in the drug therapy for oncologic diseases. In Sex and Gender Differences in Pharmacology Regitz-Zagrosek V (ed), Vol. 214, Chapter 19, pp 411–442. Springer: Berlin, Heidelberg.
Xiao G, Cao Y, Qiu X, Wang W, Wang Y (2013) Influence of gender and age on the survival of patients with nasopharyngeal carcinoma. BMC Cancer 13 (1): 226.
Xie SH, Yu IT, Tse LA, Mang OW, Yue L (2013) Sex difference in the incidence of nasopharyngeal carcinoma in Hong Kong 1983-2008: suggestion of a potential protective role of oestrogen. Eur J Cancer 49 (1): 150–155.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
OuYang, PY., Zhang, LN., Lan, XW. et al. The significant survival advantage of female sex in nasopharyngeal carcinoma: a propensity-matched analysis. Br J Cancer 112, 1554–1561 (2015). https://doi.org/10.1038/bjc.2015.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.70
Keywords
This article is cited by
-
Age-dependent changes of gender disparities in nasopharyngeal carcinoma survival
Biology of Sex Differences (2021)
-
Selectively recommend 18F-FDG PET/CT for patients with de novo nasopharyngeal carcinoma in endemic areas
Radiation Oncology (2021)
-
Efficacy and safety of intensity-modulated radiotherapy alone versus intensity-modulated radiotherapy plus chemotherapy for treatment of intermediate-risk nasopharyngeal carcinoma
Radiation Oncology (2020)
-
Distinct molecular etiologies of male and female hepatocellular carcinoma
BMC Cancer (2019)
-
Circulating tumor cells: a valuable marker of poor prognosis for advanced nasopharyngeal carcinoma
Molecular Medicine (2019)