Abstract
Background:
Adult weight gain is associated with increased risk of postmenopausal breast cancer. Most previous studies are limited by using recalled or self-reported data, and it is not known if age-specific weight changes are important for breast cancer risk.
Methods:
In a Norwegian cohort of 28 153 women (and 900 incident breast cancers) with longitudinal anthropometric measurements over up to 30 years, we studied both overall and age-related weight changes in adulthood and risk of postmenopausal breast cancer.
Results:
Overall, weight gain in adulthood was associated with increased breast cancer risk (hazard ratio (HR) per kg per year 1.31, 95% confidence interval (CI) 1.11–1.54). Weight gain before (HR per kg per year 1.38, 95% CI 1.09–1.75) or around menopause (1.69, 95% CI 1.32–2.16) was associated with increased risk, but there was no clear risk increase associated with later weight gain (HR per kg per year 0.92, 95% CI 0.73–1.18).
Conclusion:
Weight gain in adulthood was associated with increased risk of breast cancer. Our results suggest that weight gain before and around menopausal age may be particularly important for breast cancer risk among postmenopausal women.
Similar content being viewed by others
Main
The prevalence of overweight and obesity is increasing rapidly worldwide (Finucane et al, 2011). Obesity is associated with increased risk of several chronic diseases, including certain cancers (World Health Organization, 2009). For breast cancer, there has been a particularly strong and consistent risk increase associated with overweight and obesity after menopause (Friedenreich, 2001; Lahmann et al, 2004; Reeves et al, 2007; World Cancer Research Fund/American Institute for Cancer Research, 2007; Sexton et al, 2011; Anderson and Neuhouser, 2012). In contrast, there is convincing evidence that overweight and obesity before menopause are associated with reduced risk of premenopausal breast cancer (van den Brandt et al, 2000; Michels et al, 2006; World Cancer Research Fund/American Institute for Cancer Research, 2007), although this has not been found in all studies (Cecchini et al, 2012). This discrepancy between pre- and postmenopausal breast cancer is not well understood, and is further complicated by recent studies suggesting that overweight in adolescence and young adulthood may be associated with a reduced risk for both pre- and postmenopausal breast cancer (Ahlgren et al, 2004; Weiderpass et al, 2004; Bardia et al, 2008; Baer et al, 2010; Fagherazzi et al, 2012).
Weight gain in adulthood has been associated with an increased risk of postmenopausal breast cancer (Huang et al, 1997; Feigelson et al, 2004; Radimer et al, 2004; Eliassen et al, 2006; Ahn et al, 2007) and the results related to body mass at a young age seem to suggest that the increased breast cancer risk associated with obesity after menopause may be attributable to weight gain that started after reaching adulthood (Huang et al, 1997; Eliassen et al, 2006; Ahn et al, 2007). However, it is unclear whether weight changes at different ages may be particularly important for the risk of breast cancer in postmenopausal women (Friedenreich, 2001; Radimer et al, 2004; Ahn et al, 2007).
A limitation of most previous studies has been that information on changes in weight has either been baseline measurements combined with recall of weight at a younger age, or studies have only used self-reported and recalled data (Friedenreich, 2001; Anderson and Neuhouser, 2012). Both self-report (Engstrom et al, 2003) and recall are prone to misclassification and this will tend to be more profound with longer recall. Only a few and relatively small studies have had access to longitudinal measurements of body weight over a long time span (Radimer et al, 2004).
The aim of the present study was to prospectively investigate the relation between weight changes measured in adulthood and risk of postmenopausal breast cancer. The study was conducted within a large population-based cohort with anthropometric measurements conducted up to three times over >30 years. Thus, this is the largest study that has used weight measurements over a long time span to investigate associations of weight change with breast cancer risk.
Methods
Study population
The source population consists of female participants in the first two waves of the population-based Nord Trøndelag health study (HUNT) conducted in 1984–86 (HUNT1) and 1995–97 (HUNT2). The HUNT study is described in more detail elsewhere (Krokstad et al, 2012). Briefly, it consists of residents 20 years of age or older of Nord Trøndelag county in Norway. A total of 43 229 (HUNT1) and 47 177 (HUNT2) women were invited and 38 271 (88.5%, HUNT1) and 34 660 (73.5%, HUNT2) women accepted the invitation, respectively. They attended a clinical examination that included standardised measurements of height (to the nearest centimetre without shoes) and weight (to the nearest half kilogram wearing light clothes). In addition, information on education, smoking, alcohol use and leisure-time physical activity was collected using comprehensive self-administered questionnaires in both HUNT1 and HUNT2, whereas information on reproductive history (age at menarche, age at first birth and parity) and the use of postmenopausal hormone replacement therapy (HRT) was collected only in HUNT2.
All participants in the HUNT study have completed a written informed consent form specifically allowing linkage with register data.
Using the unique 11-digit identification number of Norwegian citizens, individual information from the two waves of the HUNT study was linked for those who participated in both. In addition, individual information from the two waves of the HUNT study was linked to data from a nation-wide mandatory tuberculosis screening (hereafter named as TBC survey) that was conducted between 1963 and 1975. The information from that screening includes height measured to the nearest centimetre and weight measured to the nearest kilogram on regularly calibrated scales (Tretli, 1989).
Individual information from the HUNT study was also linked to information about cancer incidence provided by the Cancer Registry of Norway (www.krefregisteret.no). The Cancer Registry of Norway has registered information on incident cancer since 1953, and the reporting of cancer is mandatory by law. The information includes date at diagnosis, clinical stage at diagnosis and histological grade. The registry information is considered to be virtually complete (Larsen et al, 2009). Breast cancer is registered according to the International Classification of Diseases 7th edition (ICD-7 code 170).
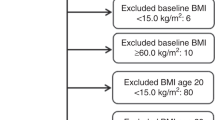
We included women for analysis who were 55 years of age or older. We chose 55 years to indicate postmenopausal status since information on menopausal status was limited. Eligible women had at least two valid measurements of body weight at age 18 years or later and were free of cancer at start follow-up. A total of 28 153 women had at least two records of weight: 21 773 women participated in the HUNT1 study and had a previous record of weight from the TBC survey, and 6380 women who participated in the HUNT2 study had a previously recorded weight from the HUNT1 study (5723) or the TBC survey (657). In addition, 13 392 of the 21 773 women who participated in the HUNT1 study had a previous record of weight from the TBC survey also participated in the HUNT2 study, and thus had weight recorded at three occasions.
The study was approved by the regional committee for medical research ethics that regulates both legal and ethical aspects of medical research in Norway.
Weight change
Since anthropometric measurements were conducted at different time intervals for each participant we constructed a variable of weight change (in kg) per year. When follow-up started at the time of the HUNT2 study, the variable was constructed to ensure maximum time between the measurements and the weight change between the TBC survey, and the HUNT2 was used whenever applicable.
Weight change was categorised into five groups; weight loss (>2.5 kg per 10 years), stable weight (±2.5 kg per 10 years), and three categories of weight gain (2.5–4.99 kg per 10 years, 5.0–7.49 kg per 10 years and ⩾7.5 kg per 10 years).
Statistical analyses
Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the association of weight change with breast cancer incidence. Each woman contributed person-time from the date of entry (see next paragraph) until the date of breast cancer diagnosis or any other cancer diagnosis, death, emigration, or to the end of follow-up (31 December 2009), whichever occurred first.
In the main analyses, women were entered after two separate weight measurements or after reaching 55 years of age whichever occurred last. Thus, 21 773 women were entered at, or after participation in the HUNT1 study and 6380 women were entered at, or after participation in the HUNT2 study.
Information on hormonal and reproductive factors (age at menarche, age at first birth, parity and HRT) that are known to influence breast cancer risk and also to be associated with overweight and obesity (Kelsey and Gammon, 1991; Bobrow et al, 2012), was collected in the HUNT2 study only. To investigate the potential confounding role of reproductive factors, separate analyses were therefore conducted for women who participated in the HUNT2 study and had at least two valid measurements of weight (19 773 women). In these analyses, women entered at the time of measurement in the HUNT2 study or at the age of 55 years, whichever occurred last.
All analyses were adjusted for age by using age as the underlying time scale and stratified by year of birth (<1920, 1920–1949 and ⩾1950) to account for possible cohort effects.
Multivariable analyses were adjusted for height at baseline (continuous), education (<10, 10–12 or >12 years), leisure-time physical activity in hours per week (<1 h light exercise (1), moderate exercise (not 1 or 3) (2) or ⩾1 h hard exercise (3)), frequency of alcohol consumption (teetotaler, <1 time last fortnight, 1–4 times last fortnight or ⩾5 times last fortnight) and smoking (never, current or former smoker) and BMI at first survey (<22, 22–25 or ⩾25). In analyses restricted to women who had participated in the HUNT2 study, we also adjusted for age at menarche (<12, 12–13 or ⩾14 years), parity and age at first birth (nulliparous, 1–2 children and <25 years at first birth, 1–2 children and ⩾25 years at first birth, ⩾3 children and <25 years at first birth, ⩾3 children and ⩾25 years at first birth).
To evaluate the influence of postmenopausal HRT on the studied associations, we also conducted stratified analyses among women who had reported the use of HRT.
Since associations of weight change with breast cancer incidence are partly determined by body size, we also did sensitivity analyses where we explored the effect of adjusting for attained BMI at last survey (<22, 22–24.9, 25–29.9 and ⩾30 kg m−2) by including BMI in the model or by stratifying on BMI at last survey (stratified Cox model).
To account for the possibility that undiagnosed breast cancer may influence weight (reversed causation), we also performed sensitivity analyses where we omitted the first 2 years of follow.
In addition to complete-case analyses, we performed multiple imputation of missing data on the possible confounders, to obtain complete data sets for the 19 772 women who participated in the HUNT2 study. Under the missing at random assumption we used the chained equations option in the multiple imputation (mi) procedure in STATA statistical software to create 50 data sets (White et al, 2011). All the variables described above, the survival time (log-transformed) and the outcome variable (incident breast cancer) were used as predictor variables together with a subset of other variables from the HUNT2 study that were judged as possible predictors for the missing data.
To evaluate potential critical age periods of weight change for the risk of breast cancer, we separately investigated weight change in three different age periods: (1) among women with a mid-age between measurements of less <45 years, (2) among women with a mid-age between measurements of 45–55 years (the period regarded as the perimenopausal phase), and (3) among women with a mid-age between measurements of 55 years or more. Mid-age was defined as the mean age between the two consecutive measurements used to construct the weight change variable (if a woman was 20 years at the time of first measurement and 35 at the second, she would have a mid-age of 27.5 years). We assessed heterogeneity of the HRs across the three strata of mid-age at weight change by a likelihood ratio test. Here, we compared models with and without a product term between mid-age at weight change (three groups) and weight change per year (continuous variable).
Among women with three consecutive measurements (n=13 392), we also evaluated the risk associated with weight change in the first period (from the TCB survey to the HUNT1 study) and the second period (from the HUNT1 study to the HUNT2 study).
Tests for linear trend were calculated per unit increase in the original continuous variable (kg per year) and reported as P-values for trend.
Proportional hazard assumptions were evaluated by log minus log plots, and showed no violation of the assumptions. All statistical tests were two-sided and all analyses were performed using STATA for Windows (Version 12 StataCorp LP, College Station, TX, USA; 1985–2007).
Results
Baseline characteristics of the participants are displayed in Tables 1A and B. In the main analysis, a total of 900 women were diagnosed with breast cancer during a mean follow-up of 12.8 years (s.d. 7.6 years), with a mean age at diagnosis of 71.3 years (s.d. 10.0 years).
Compared with women who maintained a stable weight between measurements, women who gained weight were at increased risk of breast cancer (HR per kg per year 1.31, 95% CI 1.11–1.54, P for trend 0.001) (Table 2), and adjustment for potentially confounding factors, other than baseline body height, did not substantially influence this estimate.
Women in the highest category of weight gain (equivalent to a weight gain of 7.5 kg or more over 10 years) had an HR for breast cancer of 1.63 (95% CI 1.28–2.09) compared with women with a stable weight (Table 2). In a separate analysis of women with complete information on reproductive factors in the HUNT2 study, the corresponding multivariable adjusted HR was 1.51 (95% CI 1.06–2.15) (Table 3). These findings were also consistent across strata of BMI at first survey when examined separately (data not shown).
In sensitivity analyses where we included the most recent BMI in the model, the association of weight gain with risk of breast cancer was not substantially changed (data not shown).
We assessed whether the association between weight gain and risk of breast cancer differed according to age period when the weight gain occurred. We found that weight gain before menopause (mid-age of weight change<45 years), and in the period regarded as the perimenopausal phase (mid-age of weight change between 45 and 55 years), was associated with increased risk (HR for breast cancer per kg per year 1.38 (95% CI 1.09–1.75) and 1.69 (95% CI 1.32–2.16), respectively) compared with women whose weight remained stable. In contrast, there was no clear association between weight gain and risk of breast cancer (HR for breast cancer per kg per year 0.92 (95% CI 0.73–1.18) among women who gained weight after menopause (mid-age of weight change⩾55 years) (P for heterogeneity across strata of age 0.002) (Table 4).
Results for sub-analyses among women with three consecutive measurements over 28 years (range 21–34 years) did not differ substantially from the main results (Supplementary Table 1).
Only 12% women in our analyses reported the current use of HRT at the time of the HUNT2 study, and there was a substantial proportion with missing data on this variable (Table 1B). The use of HRT was strongly and positively associated with breast cancer risk (current vs never users; HR 1.89, 95% CI 1.43–2.49). When we restricted to those who never used HRT (10 885 women and 206 cases of breast cancer), the results on weight change and breast cancer risk were similar to the overall results (data not shown). Among current users, there was no association between weight gain and risk of breast cancer; however, this analysis was limited by low statistical power (only 2322 women and 82 cases of breast cancer) (data not shown).
In analyses related to weight loss, we found no clear associations with breast cancer risk. Although the HR estimates were compatible with a slightly lower risk, the precision of the estimates was low.
Including a lag-time of minimum 2 years from the survey to entry in the analyses did not change the results (data not shown).
The results from analyses using multiple imputations to handle missing data on potential confounders were not materially different from the results of analyses restricted to participants with complete information on all variables (Table 3).
Discussion
In this population-based, prospective study of women 55 years and older, indicating postmenopausal status, we found a positive association between weight gain throughout life and risk of breast cancer. We also found that the association was stronger for women who gained weight at pre- and perimenopausal age, compared with women who gained weight later.
Our results are consistent with previous research that used recalled data on weight at younger ages (Feigelson et al, 2004; Lahmann et al, 2005; Eliassen et al, 2006; Ahn et al, 2007; World Cancer Research Fund/American Institute for Cancer Research, 2007) where, generally, a positive association between weight gain in adulthood and risk of breast cancer after menopause has been documented. Compared with most recent BMI, weight gain has been more consistently associated with increased risk in some (Feigelson et al, 2004; Ahn et al, 2007), but not all (Morimoto et al, 2002) studies. When we included weight gain and most recent BMI in the same model, BMI was not associated with risk of breast cancer, whereas the associations with weight gain remained unchanged, suggesting that weight gain may be a better measure, than a single measurement of body mass, when assessing breast cancer risk associated with body fatness among postmenopausal women. The finding is also supported by another study (Ahn et al, 2007) where the investigators found no increase in postmenopausal breast cancer risk among women with a stable high weight since the age of 18, compared with slim women.
Several authors have hypothesised that timing of weight gain could be related to breast cancer risk (Stoll, 1995; Huang et al, 1997; Friedenreich, 2001; Radimer et al, 2004). However, the epidemiological evidence was limited so far (Radimer et al, 2004; Eliassen et al, 2006; Ahn et al, 2007). In the present study, we found a stronger association among women who gained weight at pre- and perimenopausal age, compared with women who gained weight later, although this finding must be interpreted with caution. If confirmed, then this might suggest a relatively long induction period between the timing of weight gain and the clinical appearance of a tumour. Alternatively, it could reflect that the breast is more susceptible to adverse effects of weight gain in phases associated with marked hormonal changes (such as menopause) and less susceptible later in life.
The results of previous studies suggest that the positive association of body fatness with breast cancer risk is modified by the use of HRT, with a positive association evident only among non-users of HRT (Huang et al, 1997; van den Brandt et al, 2000; Morimoto et al, 2002; Feigelson et al, 2004; Lahmann et al, 2005; Eliassen et al, 2006). We attempted to confirm the possible effect modification, and our results for never users of HRT are in line with the previous research. However, our analysis among current users was limited by low statistical power.
Results from previous studies on weight loss and risk of breast cancer have shown inconsistent results, both suggesting no (Radimer et al, 2004; Ahn et al, 2007) or inverse (Harvie et al, 2005; Eliassen et al, 2006) associations. In the Nurses’ Health study (Eliassen et al, 2006) sustained weight loss was associated with reduced risk of breast cancer among postmenopausal women (who did not use HRT). Although we observed a tendency towards a lower risk with weight loss, in line with some previous studies, we found no clear evidence for an association of weight loss with breast cancer risk in the present study.
The increased risk of breast cancer in overweight and obese women has mainly been attributed to the higher conversion rates of androgenic precursors to oestradiol through increased aromatase activity in adipose tissue, resulting in increased circulating and local levels of oestrogens in overweight postmenopausal women (Friedenreich, 2001; Key et al, 2003; Key et al, 2011; Bulun et al, 2012). However, this does not fully explain that weight gain could be more strongly related to risk of postmenopausal breast cancer compared with a stable but high body weight throughout life. It has been suggested that weight gain may be a more useful marker for body fat deposition (Ballard-Barbash et al, 1997), and deposition of fat mass may be a key to understand the association of weight gain with breast cancer risk after menopause. It has also been hypothesised that breast tissue maturation is altered in women who are heavy in early life (before or during puberty) and that they, despite their earlier age at menarche, may have a prolonged maturation of breast tissue which could render the breast less susceptible to carcinogenic stimuli throughout the life (Baer et al, 2005). Recent findings suggest that girls who are heavy in puberty have lower mammographic breast density as adults (Lope et al, 2011), and mammographic density is one of the strongest known risk factors for breast cancer (McCormack and dos Santos Silva, 2006; Boyd et al, 2007).
Obesity has several other consequences that may have a role in breast cancer development. Elevated levels of insulin and insulin-like growth factor-1 are common in overweight and obese women (Gunter et al, 2009; Poole et al, 2011). Among other effects, insulin directly stimulates tumour proliferation and increases levels of free oestrogen by inhibiting liver synthesis of sex hormone binding globulins (Gunter et al, 2009). Furthermore, increased body fatness seems to render the organism in a state of chronic inflammation, which might also promote cancer development (Grivennikov et al, 2010; Harvey et al, 2011).
One may speculate whether women who are lean in childhood and adolescence and gain weight in adulthood and especially during phases of hormonal change might be more vulnerable to the adverse consequences of obesity compared with women who remained heavy since puberty or women who stay slim throughout the life.
Our study is the largest study to date that has used measured (and not recalled) data on weight and long-term changes in weight to investigate associations how weight changes may influence the risk of breast cancer. Measurements of weight are important since most studies within this area have used self-reported or recalled data, which are prone to systematic misclassification (Engstrom et al, 2003), which will also tend to be more profound with longer recall.
The HUNT study offers a possibility to adjust for a wide range of possible confounders. Still, as with any observational study, we cannot exclude the possibility of uncontrolled confounding. Nevertheless, any remaining confounder potentially able to influence our results considerably would need to (1) be strongly associated with breast cancer risk and with weight change in adulthood, and (2) be unrelated to the potential confounders that were included in our models.
The HUNT study is population based with a high attendance and the follow-up is virtually complete (0.1% loss to follow-up due to emigration from Norway) with highly reliable outcome measures (Larsen et al, 2009). Our study population was Caucasian and genetically homogeneous which on one hand may have increased the internal validity of our findings; on the other hand, our results might not directly apply to non-western populations.
Information on covariates was collected at baseline, and for some covariates (such as smoking, exercise and HRT) their value may have changed during follow-up. However, adjustment for covariates did not substantially influence the estimate of effects in the analyses. We did not have information on family history of breast cancer and could therefore not evaluate if weight change has a greater or lesser impact among women who might be at high risk due to family factors. Also, we had no information on breast tumour characteristics (such as protein expression of oestrogen (ER), progesterone (PgR) receptor or HER-2) and could not explore whether our findings may differ by breast cancer subtype.
In conclusion, we found that weight gain in adulthood is positively associated with breast cancer risk in women older than 55 years. Our results suggest that this positive association might be stronger for women who gain weight at pre- and perimenopausal age, compared with women who gain weight later. However, this finding should be interpreted with caution.
Because weight gain is one of the few modifiable risk factors for breast cancer, weight control provides an important possibility for prevention of breast cancer in postmenopausal women.
Change history
03 September 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI (2004) Growth patterns and the risk of breast cancer in women. N Engl J Med 351 (16): 1619–1626.
Ahn J, Schatzkin A, Lacey JV Jr, Albanes D, Ballard-Barbash R, Adams KF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann MF (2007) Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med 167 (19): 2091–2102.
Anderson GL, Neuhouser ML (2012) Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res 5 (4): 515–521.
Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, Willett WC (2005) Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res 7 (3): R314–R325.
Baer HJ, Tworoger SS, Hankinson SE, Willett WC (2010) Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol 171 (11): 1183–1194.
Ballard-Barbash R, Birt DF, Kestin M, King IB (1997) Perspectives on integrating experimental and epidemiologic research on diet, anthropometry and breast cancer. J Nutr 127 (5 Suppl): 936S–939S.
Bardia A, Vachon CM, Olson JE, Vierkant RA, Wang AH, Hartmann LC, Sellers TA, Cerhan JR (2008) Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 17 (2): 374–378.
Bobrow KL, Quigley MA, Green J, Reeves GK, Beral V (2012) Persistent effects of women's parity and breastfeeding patterns on their body mass index: results from the Million Women Study. Int J Obes (Lond) 37 (5): 712–717.
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356 (3): 227–236.
Bulun SE, Chen D, Moy I, Brooks DC, Zhao H (2012) Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 23 (2): 83–89.
Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Land SR, Weissfeld JL, Wolmark N (2012) Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res 5 (4): 583–592.
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE (2006) Adult weight change and risk of postmenopausal breast cancer. JAMA 296 (2): 193–201.
Engstrom JL, Paterson SA, Doherty A, Trabulsi M, Speer KL (2003) Accuracy of self-reported height and weight in women: an integrative review of the literature. J Midwifery Womens Health 48 (5): 338–345.
Fagherazzi G, Guillas G, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S (2012) Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev 22: 29–37.
Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE (2004) Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev 13 (2): 220–224.
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377 (9765): 557–567.
Friedenreich CM (2001) Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev 10 (1): 15–32.
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140 (6): 883–899.
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD (2009) Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 101 (1): 48–60.
Harvey AE, Lashinger LM, Hursting SD (2011) The growing challenge of obesity and cancer: an inflammatory issue. Ann NY Acad Sci 1229: 45–52.
Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA (2005) Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev 14 (3): 656–661.
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC (1997) Dual effects of weight and weight gain on breast cancer risk. JAMA 278 (17): 1407–1411.
Kelsey JL, Gammon MD (1991) The epidemiology of breast cancer. CA Cancer J Clin 41 (3): 146–165.
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE Jr., Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR Endogenous Hormones Breast Cancer Collaborative G (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95 (16): 1218–1226.
Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, Hankinson SE, Tworoger SS, Koenig K, Zeleniuch-Jacquotte A, Arslan AA, Toniolo P, Shore RE, Krogh V, Micheli A, Berrino F, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Lui LY, Cummings SR, Gunter MJ, Rohan TE, Strickler HD (2011) Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105 (5): 709–722.
Krokstad S, Langhammer A, Hveem K, Holmen T, Midthjell K, Stene T, Bratberg G, Heggland J, Holmen J (2012) Cohort profile: the HUNT Study, Norway. Int J Epidemiol e-pub ahead of print 9 August 2012; doi:10.1093/ije/dys095.
Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, Berrino F, Tjonneland A, Bigaard J, Olsen A, Overvad K, Clavel-Chapelon F, Nagel G, Boeing H, Trichopoulos D, Economou G, Bellos G, Palli D, Tumino R, Panico S, Sacerdote C, Krogh V, Peeters PH, Bueno-de-Mesquita HB, Lund E, Ardanaz E, Amiano P, Pera G, Quiros JR, Martinez C, Tormo MJ, Wirfalt E, Berglund G, Hallmans G, Key TJ, Reeves G, Bingham S, Norat T, Biessy C, Kaaks R, Riboli E (2004) Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int J Cancer 111 (5): 762–771.
Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjonneland A, Olsen A, Overvad K, Key TJ, Allen NE, Khaw KT, Bingham S, Berglund G, Wirfalt E, Berrino F, Krogh V, Trichopoulou A, Lagiou P, Trichopoulos D, Kaaks R, Riboli E (2005) Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC). Br J Cancer 93 (5): 582–589.
Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Moller B (2009) Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 45 (7): 1218–1231.
Lope V, Perez-Gomez B, Moreno MP, Vidal C, Salas-Trejo D, Ascunce N, Roman IG, Sanchez-Contador C, Santamarina MC, Carrete JA, Collado-Garcia F, Pedraz-Pingarron C, Ederra M, Ruiz-Perales F, Peris M, Abad S, Cabanes A, Pollan M, Spain DDM (2011) Childhood factors associated with mammographic density in adult women. Breast Cancer Res Treat 130 (3): 965–974.
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15 (6): 1159–1169.
Michels KB, Terry KL, Willett WC (2006) Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med 166 (21): 2395–2402.
Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, Stefanick ML, McTiernan A (2002) Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control 13 (8): 741–751.
Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ (2011) Body size in early life and adult levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3. Am J Epidemiol 174 (6): 642–651.
Radimer KL, Ballard-Barbash R, Miller JS, Fay MP, Schatzkin A, Troiano R, Kreger BE, Splansky GL (2004) Weight change and the risk of late-onset breast cancer in the original Framingham cohort. Nutr Cancer 49 (1): 7–13.
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D Million Women Study C (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335 (7630): 1134.
Sexton KR, Franzini L, Day RS, Brewster A, Vernon SW, Bondy ML (2011) A review of body size and breast cancer risk in Hispanic and African American women. Cancer 117 (23): 5271–5281.
Stoll BA (1995) Timing of weight gain in relation to breast cancer risk. Ann Oncol 6 (3): 245–248.
Tretli S (1989) Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int J Cancer 44 (1): 23–30.
van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ (2000) Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 152 (6): 514–527.
Weiderpass E, Braaten T, Magnusson C, Kumle M, Vainio H, Lund E, Adami HO (2004) A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 13 (7): 1121–1127.
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30 (4): 377–399.
World Cancer Research Fund/American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and The Prevention of Cancer: A Global Perspective vol. 2012. AICR: Washington, DC.
World Health Organization (2009) Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks vol. 2013. WHO: Geneva.
Acknowledgements
The Nord-Trøndelag Health Study (the HUNT study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Alsaker, M., Janszky, I., Opdahl, S. et al. Weight change in adulthood and risk of postmenopausal breast cancer: the HUNT study of Norway. Br J Cancer 109, 1310–1317 (2013). https://doi.org/10.1038/bjc.2013.403
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.403
Keywords
This article is cited by
-
Lipoprotein and metabolite associations to breast cancer risk in the HUNT2 study
British Journal of Cancer (2022)
-
Young adulthood body mass index, adult weight gain and breast cancer risk: the PROCAS Study (United Kingdom)
British Journal of Cancer (2020)
-
World Cancer Research Fund International: Continuous Update Project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk
Cancer Causes & Control (2019)
-
Midlife weight gain is a risk factor for obesity-related cancer
British Journal of Cancer (2018)
-
Weight and weight changes throughout life and postmenopausal breast cancer risk: a case-control study in France
BMC Cancer (2016)