Abstract
Background:
The mesenchymal-epithelial transition factor (MET) receptor is dysregulated in hepatocellular carcinoma (HCC), and tivantinib (ARQ 197) is an oral, selective, MET inhibitor.
Methods:
This Phase-1b study assessed tivantinib safety as primary objective in patients with previously treated HCC and Child-Pugh A or B liver cirrhosis. Patients received oral tivantinib 360 mg twice daily until disease progression or unacceptable toxicity.
Results:
Among 21 HCC patients, common drug-related adverse events (AEs) were neutropaenia, anaemia, asthenia, leucopaenia, anorexia, diarrhoea, and fatigue. No drug-related worsening of liver function or performance status occurred, but one Child-Pugh B patient experienced drug-related bilirubin increase. Four patients had drug-related serious AEs, including one neutropaenia-related death. Haematologic toxicities were more frequent than in previous tivantinib studies but were manageable with prompt therapy. Best response was stable disease (median, 5.3 months) in 9 of 16 evaluable patients (56%). Median time to progression was 3.3 months.
Conclusion:
Tivantinib demonstrated a manageable safety profile and preliminary antitumour activity in patients with HCC and Child-Pugh A or B cirrhosis.
Similar content being viewed by others
Main
The current standard of care for patients with advanced hepatocellular carcinoma (HCC) is systemic treatment with sorafenib (Bruix and Sherman, 2011; EASL-EORTC, 2012; Forner et al, 2012). Sorafenib significantly improved overall survival compared with placebo in two large, randomised, phase 3 trials (Llovet et al, 2008; Cheng et al, 2009). There is no standard therapy for patients who are intolerant of sorafenib or experience disease progression after sorafenib treatment (NCCN, 2011).
The hepatocyte growth factor (HGF)/mesenchymal-epithelial transition factor (MET) receptor tyrosine kinase pathway is frequently dysregulated in human cancers and has a critical role in the pathophysiology of HCC (Boix et al, 1994; Wang et al, 2001; Xie et al, 2010). In HCC, activation of the HGF/MET pathway is associated with an aggressive phenotype and poor prognosis (Kaposi-Novak et al, 2006). Tivantinib (ARQ 197) is an oral, selective, MET inhibitor that disrupts MET-dependent downstream signalling by inhibiting constitutive and HGF-mediated MET phosphorylation (Munshi et al, 2010). Tivantinib inhibits MET activation in human HCC and other tumour cell lines and has demonstrated antitumour activity in human tumour xenograft models (Munshi et al, 2010; Previdi et al, 2012; Salvi et al, 2007). In clinical studies, tivantinib appears to be well tolerated and has antitumour activity as monotherapy or in combination with other agents (Rosen et al, 2011; Sequist et al, 2011; Yap et al, 2011).
In dose-escalation studies, the recommended phase 2 dose (RP2D) of tivantinib was defined as 360 mg twice daily (BID) (Rosen et al, 2011; Yap et al, 2011). However, these studies did not include many patients with HCC and chronic liver disease, which may affect drug metabolism (Verbeeck, 2008). This phase 1 study evaluated the safety of tivantinib in patients with documented HCC and liver cirrhosis, focusing especially on the effect of tivantinib on liver function.
Materials and methods
Patients
Adult patients (⩾18 years of age) with histologically or cytologically confirmed advanced HCC, Barcelona Clinic Liver Cancer (BCLC) stage A–C (Forner et al, 2012), and Child-Pugh A cirrhosis with no clinical ascites were eligible (see Online Supplementary Information for additional eligibility requirements). The protocol was later amended to include patients with Child-Pugh B cirrhosis (without ascites at physical examination) and to allow validated non-pathologic HCC diagnosis ((Bruix and Sherman, 2005; Forner et al, 2008). This open-label, single-arm study (ClinicalTrials.gov ID: NCT00802555) was conducted in accordance with the Declaration of Helsinki and good clinical practice, and all patients provided written informed consent.
Study design and treatment
Oral tivantinib (360 mg BID) was administered 1 h before or 2 h after eating in 28-day treatment cycles. Dose reductions (up to 2) were allowed in patients with grade 3 or 4 drug-related adverse events (AEs) with no dose re-escalation (see Online Supplementary Information). The primary objective was safety, with the goal of defining the RP2D in cirrhotic HCC patients. If during the first month of treatment no more than two of 25 patients experienced a drug-related decrease in liver function, hepatic AE, or two-point decline in performance status, 360 mg BID would be the RP2D (see Online Supplementary Information). Secondary objectives included time to progression (TTP), objective response rate, disease control rate, and tivantinib pharmacokinetics.
Patient assessments
Adverse events were assessed using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (CTCAE, 2006). Clinically significant laboratory abnormalities were reported as AEs. The efficacy population included all patients who received at least one complete cycle of therapy and had at least one post-baseline tumour assessment. Tumour response was assessed using Response Evaluation Criteria In Solid Tumours version 1.1 every 8 weeks until disease progression, unacceptable toxicity, death, or study withdrawal.
Pharmacokinetic assessments
Blood samples (6 ml) for 24 h pharmacokinetic analysis were collected on study days 1/2 and 15/16 (see Online Supplementary Information). Plasma concentration-time data were analysed by non-compartmental methods using WinNonLin 4.0 (Pharsight Corporation, Mountain View, CA, USA).
Statistical analysis
Data were analysed using standard statistical methods (see Online Supplementary Information).
Results
Demographics and baseline characteristics
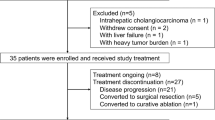
Twenty-one patients were enrolled between March 2009 and November 2010, of whom 19 (91%) had received previous sorafenib therapy and seven had received more than one previous therapy. Demographics and baseline characteristics are shown in Table 1. Four patients had Child-Pugh B status (with ⩽8 points), eight had distant metastases, and seven had vascular invasion at study entry. Median baseline plasma alpha-fetoprotein level was 234 ng ml−1 (range, 2–63,918 ng ml−1) and was higher than 200 ng ml−1 in 11 patients.
Treatment duration and dose modifications
At time of analysis, all patients had discontinued study treatment: 17 (81%) because of radiographic or clinical disease progression, and four (19%) because of AEs. Overall, 16 patients (76%) received at least two cycles of study drug (range, 1–15 cycles), and median treatment duration was 1.8 months (range, 0.1–15.9 months). Nine patients (43%) were treated for >2 months; two patients continued to receive treatment for 1.5 months and >24 months, respectively, after confirmed radiographic disease progression because of continued clinical benefit. Five patients (24%) required tivantinib dose reductions because of AEs, and eight (38%) required dose interruptions (<2 weeks) because of AEs and subsequently were able to resume treatment at the same dose.
Safety and tolerability
All patients received at least one dose of tivantinib and were evaluable for safety. The most common drug-related AEs were neutropaenia, anaemia, leucopaenia, asthenia, anorexia, diarrhoea, and fatigue (Table 2). Grade 3 or greater drug-related AEs were observed in 11 patients (52%), including neutropaenia in eight patients (38%). Neutropaenia was the primary reason for dose reduction, interruption, or discontinuation.
Serious AEs that were definitely, probably, or possibly related to tivantinib in the opinion of the investigator were reported in four patients (19%). These included grade 3 anaemia (n=1); grade 3 anaemia and grade 4 neutropaenia (n=1); grade 4 leucopaenia and grade 4 neutropaenia (n=1); and grade 4 leucopaenia, grade 4 neutropaenia, and grade 5 septic shock (n=1). Septic shock secondary to neutropaenia was the only drug-related AE leading to death.
A total of 77 myelosuppression events considered related to study drug were reported in 14 patients. Additionally, four cardiac events were reported that were considered possibly or probably related to study drug. No patient experienced drug-related worsening of liver function or decreased Eastern Cooperative Oncology Group performance status during the first cycle, except for one patient with Child-Pugh B cirrhosis who experienced elevated bilirubin that was considered possibly drug related.
Pharmacokinetics
The plasma concentration-time profile of tivantinib was characterised by mean peak levels occurring 4 h and 2 h post dose on days 1 and 15, respectively (Supplementary Figure S1). However, considerable interpatient variability (coefficient of variation range, 43–73%) was observed in tivantinib pharmacokinetic parameters (Supplementary Table S1). Significant tivantinib accumulation in plasma was observed after multiple oral doses.
Tumour response
Sixteen of 21 patients (46%) were evaluable for tumour response. Among five non-evaluable patients (24%), three had no post-therapy tumour assessment and two had not completed one full cycle of treatment at the time of confirmed disease progression. No objective responses were reported. Best response of stable disease was observed in nine of 16 evaluable patients (56%; Figure 1). A tumour reduction of ∼20% was observed in one patient who experienced prolonged stable disease. Median TTP was 3.3 months (range, 1.7–5.3 months) in the evaluable population (Supplementary Figure S2) and 1.8 months (range, 1.6–5.3 months) in the intent-to-treat population.
Discussion
Oral tivantinib was found to have a manageable safety profile (based on prospectively defined liver function criteria) and preliminary antitumour activity in patients with HCC and Child-Pugh A or B liver cirrhosis (up to 7 points), and 360 mg BID was considered the RP2D in this patient population. These results are encouraging and support further investigation of tivantinib in this setting. Neutropaenia, which occurred primarily within the first 30 days of treatment, was generally manageable with prompt dose modification, growth factors, and antibiotics. However, the frequency of grade 3 or greater neutropaenia (38%) was higher than in previous single-agent, phase 1, dose-ranging studies, in which grade 3 or greater neutropaenia occurred in <5% of patients (Rosen et al, 2011; Yap et al, 2011). Moreover, one patient in the current study died from septic shock secondary to neutropaenia. In a subsequent phase 2 study in previously treated patients with HCC and Child-Pugh A cirrhosis, the tivantinib dose was reduced to 240 mg BID and a modified dose-reduction schema was implemented because of grade 3 or greater neutropaenia (Santoro et al, 2012).
Pharmacokinetic data indicated plasma accumulation of tivantinib after multiple doses, with an area under the plasma concentration-time curve among HCC patients approximately two-fold higher than in patients with other solid tumours, but there was high interpatient variability. Although data are limited, there was no indication of a link between drug-related AEs and dose, tivantinib exposure, or Child-Pugh status, or a link between baseline demographic variables and tivantinib exposure. However, a population pharmacokinetics analysis subsequently showed that tivantinib exposure correlated with the incidence of grade 3 or greater neutropaenia (Zahir et al, 2012). These findings are consistent with evidence that tivantinib is extensively metabolised in the liver (Bagai et al, 2010; Bathala et al, 2012). Thus, a tivantinib dose of 240 mg BID with careful monitoring of haematologic toxicity is recommended in HCC patients with liver cirrhosis.
Change history
15 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bagai R, Fan W, Ma PC (2010) ARQ-197, an oral small molecule inhibitor of c-Met for the treatment of solid tumors. IDrugs 13 (6): 404–414
Bathala MS, Nakai D, Murai T, Pickersgill F, Zahir H, Tokui T (2012) Absorption, distribution, metabolism, and excretion of 14C-labeled tivantinib (ARQ 197) in healthy male subjects. 103rd Annual Meeting of the American Association for Cancer Research 31 Mar–4 Apr 2012; Chicago, IL. Abstract 747
Boix L, Rosa JL, Ventura F, Castells A, Bruix J, Rodes J, Bartrons R (1994) c-met mRNA overexpression in human hepatocellular carcinoma. Hepatology 19 (1): 88–91
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42 (5): 1208–1236
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53 (3): 1020–1022
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10 (1): 25–34
CTCAE (2006) Common Terminology Criteria for Adverse Events version 3.0 http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
EASL-EORTC (2012) European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 56 (4): 908–943
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379 (9822): 1245–1255
Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, Bru C, Bruix J (2008) Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 47 (1): 97–104
Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS (2006) Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest 116 (6): 1582–1595
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359 (4): 378–390
Munshi N, Jeay S, Li Y, Chen CR, France DS, Ashwell MA, Hill J, Moussa MM, Leggett DS, Li CJ (2010) ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther 9 (6): 1544–1553
NCCN (2011) National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Hepatobiliary Cancers, version 2.1012 http://www.nccn.org
Previdi S, Abbadessa G, Dalo F, France DS, Broggini M (2012) Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. Mol Cancer Ther 11 (1): 214–223
Rosen LS, Senzer N, Mekhail T, Ganapathi R, Chai F, Savage RE, Waghorne C, Abbadessa G, Schwartz B, Dreicer R (2011) A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res 17 (24): 7754–7764
Salvi A, Arici B, Portolani N, Giulini SM, De Petro G, Barlati S (2007) In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int J Oncol 31 (2): 451–460
Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlieberghe H, Trojan J, Kolligs FT, Weiss A, Miles S, Gasbarrini A, Lencioni M, Cicalese L, Sherman M, Gridelli C, Buggish P, Gerken G, Schmid RM, Boni C, Personeni N, Hassoun Z, Abbadessa G, Schwartz B, Von Roemeling R, Lamar ME, Chen Y, Porta C (2012) Efficacy and safety of tivantinib as second-line therapy for advanced hepatocellular carcinoma: a phase 2, randomized, placebo-controlled study. Lancet Oncol e-pub ahead of print 19 November 2012; doi: 10.1016/S1470-2045(12)70490-4
Sequist LV, von Pawel J, Garmey EG, Akerley WL, Brugger W, Ferrari D, Chen Y, Costa DB, Gerber DE, Orlov S, Ramlau R, Arthur S, Gorbachevsky I, Schwartz B, Schiller JH (2011) Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 29 (24): 3307–3315
Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 64 (12): 1147–1161
Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM (2001) Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 153 (5): 1023–1034
Xie B, Xing R, Chen P, Gou Y, Li S, Xiao J, Dong J (2010) Down-regulation of c-Met expression inhibits human HCC cells growth and invasion by RNA interference. J Surg Res 162 (2): 231–238
Yap TA, Olmos D, Brunetto AT, Tunariu N, Barriuso J, Riisnaes R, Pope L, Clark J, Futreal A, Germuska M, Collins D, deSouza NM, Leach MO, Savage RE, Waghorne C, Chai F, Garmey E, Schwartz B, Kaye SB, de Bono JS (2011) Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol 29 (10): 1271–1279
Zahir H, Kastrissios H, Carothers T, Jansen M, Mendel J, Savage R, Abbadessa G, Chai F, Schwartz B, Miller R, Tokui T (2012) Exposure-response relationship to assess the risk of neutropenia in patients with hepatocellular carcinoma (HCC) treated with tivantinib. Ann Oncol 23 (suppl 9): ix244–ix245, Abstract 738P
Acknowledgements
We are grateful to the patients participating in this study and to their family members. We thank Bret Wing, PhD, ProEd Communications, Inc., for his medical editorial assistance with this manuscript. This work was partially supported by a grant from the Instituto de Salud Carlos III (PI 11/01830), and CR-L is partially supported by a grant from Instituto de Salud Carlos III (FI09/00510). The BCLC is funded through the Spanish Biomedical Research Network (CIBER) for the area of Hepatic and Digestive disorders. Financial support for the study and medical editorial assistance was provided by ArQule, Inc., Woburn, MA, USA; and Daiichi Sankyo, Inc., a member of the Daiichi Sankyo Group; Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Author contributions
AS contributed to study design. AS, CR-L, MS, PZ, and LR contributed to provision and management of study patients, data collection, data interpretation, and writing. JB was the Principal Investigator of the study and contributed to the study design, data interpretation, and writing. LC, AG, NS, ML, RS, and GA contributed to data analysis and manuscript editing. BS contributed to study design, data interpretation, data analysis, and manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JB is a consultant for Abbott, AngioDynamics, ArQule, Bayer, BioAlliance, Biocompatibles, BMS, Eisai, GlaxoSmithKline, ImClone, Jennerex, Kowa, Lilly, MedImmune, Novartis, OSI, Pharmexa, Roche, Sanofi, Schering-Plough, and Sumitomo. AS is a consultant for ArQule and Bayer. GA, ML, BS, and RS are employees of ArQule. LR received travel grants from ArQule. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Santoro, A., Simonelli, M., Rodriguez-Lope, C. et al. A Phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer 108, 21–24 (2013). https://doi.org/10.1038/bjc.2012.556
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.556
Keywords
This article is cited by
-
Silencing CircEIF3I/miR-526b-5p Axis Epigenetically Targets HGF/c-Met Signal to Hinder the Malignant Growth, Metastasis and Angiogenesis of Hepatocellular Carcinoma
Biochemical Genetics (2023)
-
c-Met up-regulates the expression of PD-L1 through MAPK/NF-κBp65 pathway
Journal of Molecular Medicine (2022)
-
A phase I trial of topotecan plus tivantinib in patients with advanced solid tumors
Cancer Chemotherapy and Pharmacology (2018)
-
Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors
Investigational New Drugs (2015)
-
Advances in managing hepatocellular carcinoma
Frontiers of Medicine (2014)