Abstract
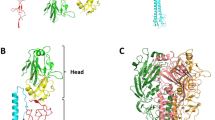
The structure of a complex of influenza hemagglutinin (HA) with a neutralizing antibody shows that the antibody binds to HA at a distance from the virus receptor binding site. Comparison of the properties of this antibody and its Fab with those of an antibody that recognizes an epitope overlapping the receptor binding site leads to two main conclusions. First, inhibition of receptor binding is an important component of neutralization. Second, the efficiency of neutralization by the antibodies ranks in the same order as their avidities for HA, and their large size makes these antibodies highly efficient at neutralization, regardless of the location of their epitope in relation to the virus receptor binding site. These observations provide rationales for the range of antibody specificities that are detected in immune sera and for the distribution of sequence changes on the membrane-distal surface of influenza HAs that occur during 'antigenic drift.'
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Wiley, D.C. & Skehel, J.J. Ann. Rev. Biochem. 56, 365–394 (1987).
Fitch, W.M., Bush, R.M., Bender, C.A. & Cox, N.J. Proc. Natl. Acad. Sci. USA 94, 7712–7718 (1997).
Wiley, D.C., Wilson, I.A. & Skehel, J.J. Nature 289, 373–378 (1981).
Fleury, D., Wharton, S.A., Skehel, J.J., Knossow, M. & Bizebard, T. Nature Struct. Biol. 5, 119–123 (1998).
Knossow, M., Daniels, R.S., Douglas, A.R., Skehel, J.J. & Wiley, D.C. Nature 311, 678–680 (1984).
Bernstein, F.C. et al. J. Mol. Biol. 112, 535–542 (1977).
Wrigley, N.G. et al. Virology 131, 308–314 (1983).
Bizebard, T., et al. Nature 376, 92–94 (1995).
Skehel, J.J., et al. In Molecular approaches to the control of infectious diseases (eds Brown, F, Burton, D, Doherty, P, Mekalanos, J. & Norrby E.) 1–7 (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York; 1997).
Davies, D.R. & Chacko, S. Acc. Chem. Res. 26, 421–427 (1993).
Wilson, I.A., Skehel, J.J. & Wiley, D.C. Nature 289, 366–373 (1981).
Weis, W. et al. Nature 333, 426–431 (1988).
Friguet, B., Djavadi-Ohaniance, L. & Goldberg, M.E. In Protein structure: a practical approach (ed. Creighton T.E.) 287–309 (IRL Press, Oxford, UK; 1989).
Weis, W., Brünger, A., Skehel, J. & Wiley, D. J. Mol. Biol. 212, 737–761 (1990).
Smith, T.J., Chase, E.S., Schmidt, T.J., Olson, N.H. & Baker, T.S. Nature 383, 350–354 (1996).
Cusack, S., Ruigrok, R.W.H., Krygsman, P.C.J. & Mellema, J.E. J. Mol. Biol. 186, 565–582 (1985).
Roost, H.-P. et al. Proc. Natl. Acad. Sci. USA 92, 127–1261 (1995).
Lamarre, A. & Talbot, P.J. J. Immunol. 154, 3975–3984 (1995).
Parren, P.W.H.I. et al. J. Virol. 72, 3512–3519 (1998).
Schofield, D.J., Stephenson, J.R. & Dimmock, N.J. J. Gen. Virol. 78, 2431–2439 (1997).
Ruigrok, R.W. et al. J. Gen. Virol. 69, 2785–2795 (1988).
Bizebard, T. et al. J. Mol. Biol. 216, 513–514 (1990).
Gigant, B., Fleury, D., Bizebard, T., Skehel, J.J. & Knossow, M. Proteins Struct. Funct. Genet. 23, 115–117 (1995).
Navaza, J. Acta. Crystallogr. A 50, 157–163 (1994).
Brünger, A.T. X-PLOR(Version 3.1) manual. Yale University Press, New Haven, Connecticut; 1992).
Jones, T.A., Zhou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Laskowski, R.A., McArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Sambrook, J., Maniatis, T. & Fritsch, E.F. Molecular cloning, a laboratory manual. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York; 1989).
Jones, S.T. & Bendig, M.M. Bio/Technology 9, 88–89 (1991).
Gleeson, T.J. & Staden, R. Comput. Appl. Biosci. 7, 398 (1991).
Karlsson, R., Michaelsson, A. & Mattsson, L. J. Immunol. Methods 145, 229–240 (1991).
Friguet, B., Chaffotte, A., Djavadi-Ohaniance, L. & Goldberg, M.E. J. Immunol. Methods 77, 305–319 (1985).
Appleyard, G. & Maber, H.B. J. Gen. Virol. 25, 351–357 (1974).
Kraulis, P. J. Appl. Crystallogr. 24, 924–950 (1991).
Acknowledgements
We thank the staff of the LURE and ESRF for their help during data collection, and A. Douglas, B. Gigant, R. Gonsalves and D. Stevens for excellent assistance. This work was supported by a grant from the EU BIOMED program.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleury, D., Barrère, B., Bizebard, T. et al. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site . Nat Struct Mol Biol 6, 530–534 (1999). https://doi.org/10.1038/9299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9299
This article is cited by
-
Potential neutralizing antibodies discovered for novel corona virus using machine learning
Scientific Reports (2021)
-
A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA
Nature Communications (2018)
-
A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action
Nature Communications (2017)
-
Serologic screenings for H7N9 from three sources among high-risk groups in the early stage of H7N9 circulation in Guangdong Province, China
Virology Journal (2014)
-
Antiviral strategies against influenza virus: towards new therapeutic approaches
Cellular and Molecular Life Sciences (2014)