Abstract
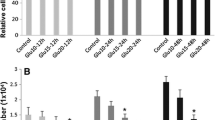
An unusual amino acid, hypusine [N ε-(4-amino-2-hydroxybutyl)lysine], is formed post-translationally in a single cellular protein, the eukaryotic translation initiation factor 5A (eIF5A) by deoxyhypusine synthase and deoxyhypusine hydroxylase. Although eIF5A and its hypusine modification are essential for eukaryotic cell viability, the true physiological function of eIF5A is yet unknown. We have examined the effects of N 1-guanyl-1,7-diaminoheptane (GC7), a potent inhibitor of deoxyhypusine synthase, on endothelial cell proliferation, differentiation and apoptosis. Upon treatment of human umbilical vein endothelial cells (HUVEC) with GC7, dose-dependent inhibition of hypusine formation and cellular proliferation was observed. GC7 at 10 μM caused almost complete inhibition of cellular hypusine synthesis and led to cytostasis of HUVEC. Pretreatment of HUVEC with GC7 up to 50 μM for 4 days had little effect on the attachment and differentiation of these cells on Matri-gel and did not cause induction of apoptosis. Instead, the GC7 pretreatment (96 h at 5–50 μM) elicited protective effects against apoptotic death of HUVEC induced by serum starvation. These results suggest that eIF-5A may be involved in expression of proteins essential for apoptosis of endothelial cells as well as those for cellular proliferation.
Similar content being viewed by others
References
Park MH, Wolff EC, Folk JE: Hypusine; its-posttranslational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors 4: 95–104, 1993
Chen KY, Liu AY: Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals 6: 105–109, 1997
Park MH, Lee YB, Joe YA: Hypusine is essential for eukaryotic cell proliferation. Biol Signals 6: 115–123, 1997
Murphey RJ, Gerner EW: Hypusine formation in protein by a two-step process in cell lysates. J Biol Chem 262: 15033–15036, 1987
Park MH, Wolff EC: Cell-free synthesis of deoxyhypusine; separation of protein substrate and enzyme and identification of 1,3–diaminopropane as a product of spermidine cleavage. J Biol Chem 263: 15264–15269, 1988
Abbruzzese A, Park MH, Folk JE: Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem 261: 3085–3089, 1986
Duncan RF, Hershey JWB: Changes in eIF-4D hypusine modification or abundance are not correlated with translational repression in HeLa cells. J Biol Chem 261: 12903–12906, 1986
Park, MH: Regulation of biosynthesis of hypusine in Chinese hamster ovary cells. Evidence for eIF-4D precursor polypeptides. J Biol Chem 262: 12730–12734, 1987
Smit-McBride X, Schnier J, Kaufman RJ, Hershey JWB: Protein synthesis initiation factor eIF-4D. Functional comparison of native and unhypusinated forms of the protein. J Biol Chem 264: 18527–18530, 1989
Park, MH: The essential role of hypusine in eukaryotic translational initiation factor 4D (eIF-4D). Purification of eIF-4D and its precursors and comparison of their activities. J Biol Chem 264: 18531–18535, 1989
Park MH, Cooper HL, Folk JE: Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci USA 78: 2869–2873, 1981
Byers TL, Ganem B, Pegg AE: Cytostasis induced in L1210 murine leukemia cells by the S-adenosyl-L-methionine decarboxylase inhibitor 5'-{[(Z)-4–amino-2–butenyl]methylamino}-5′-deoxyadenosine may be due to hypusine depletion. Biochem J 287: 717–724, 1992
Park MH, Wolff EC, Lee YB, Folk JE: Antiproliferative effects of inhibitors of deoxyhypusine synthase. J Biol Chem 269: 27827–27832, 1994
Hanauske-Abel HM, Park M-H, Hanauske A-R, Popowicz AM, Lalande M, Folk JE: Inhibition of G1–S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochem Biophys Acta 1221: 115–124, 1994
Schnier J, Schwelberger H, Smit-McBride Z, Kang HA, Hershey JWB: Translation initiation factor 5A and its hypusine modification are essential for cell viability in yeast. Mol Cell Biol 11: 3105–3114, 1991
Wohl T, Klier H, Ammer H, Lottspeich G, Magdolen V: The HYP2 gene of Saccharomyces cerevisiae is essential for aerobic growth: Characterization of different isoforms of the hypusine-containing protein Hyp2p and analysis of gene disruption mutants. Mol Gen Genet 241: 305–311, 1993
Sasaki K, Abid MR, Miyazaki MV: Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett 384: 151–154, 1996
Park MH, Joe YA, Kang KR: Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J Biol Chem 273: 1677–1683, 1998
Kang HA, Hershey JWB: Effects of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem 269: 3934–3940, 1994
Ruhl M, Himmelspach M, Bahr GM, Himmerschmid F, Jaksche H, Wolff B, Aschuer H, Farrington GK, Probst H, Bevec D, Hauber J: Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type I Rev activation domain mediating trans-activation. J Cell Biol 123: 1309–1320, 1993
Katahira J, Ishizaki T, Sakai H, Adachi A, Yamamoto K, Shida H: Effects of translational initiation factor eIF-5A on the functioning of human T-cell leukemia virus Type I Rex and human immunodeficiency virus Rev inhibited trans-dominantly by a Rex mutant deficient in RNA binding. J Virol 69: 3125–3133, 1995
Zuk D, Jacobson A: A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J 17: 2914–2925, 1998
Jakus J, Wolff EC, Park MH, Folk JE: Features of the spemidine-binding site of deoxyhypusine synthase as derived from inhibitor studies. J Biol Chem 268: 13151–13159, 1993
Lee YB, Park MH, Folk JE: Diamine and triamine analogs and derivatives as inhibitors of deoxyhypusine synthase: synthesis and biological activity. J Med Chem 38: 3053–3061, 1995
Shi X-P, Yin K-C, Ahern J, Davis LJ, Stern AM, Waxman L: Effects of N 1-guanyl-1,7–diaminoheptane, an inhibitor of deoxyhypusine synthase, on the growth of tumorigenic cell lines in culture. Biochim Biophys Acta 1310: 119–126, 1996
Jaffe EA, Nachman RL, Becker CG, Minick R: Culture of human endothelial cells derived from umbilical veins. J Clin Invest 52: 2745–2756, 1973
Seiler N: In P.P. McCann, A.E. Pegg, A. Sjoerdsma (eds). Inhibition of Polyamine Metabolism, Biological Significance and Basis for New Therapies. Academic Press, Orlando, 1987, pp 49–78
Araki S, Shimada Y, Kaji K, Hayashi H: Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun 168: 1194–1200, 1990
Gerber H-P, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N: Vascular endothelial growth factor regulates endothelial cell survival through thephosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998
Benne R, Brown-Luede M, Hershey JWB: Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J Biol Chem 253: 3070–3077, 1978
Gerner EW, Mamont PS, Bernhardt A, Siat M: Post-translational modification of the protein-synthesis initiation factor eIF-4D by spermidine in rat hepatoma cells. Biochem J 239: 379–386, 1986
Zimrin AB, Villeponteau, Maciag T: Models of in vitro angiogenesis: endothelial cell differentiation on fibrin but not matrigel is transcriptionally dependent. Biochem Biophys Res Commun 213: 630–638, 1995
Chen ZP, Yan YP, Ding QJ, Knapp S, Potenza JA, Schugar HJ, Chen KY: Effects of inhibitors of deoxyhypusine synthase on the differentiation of mouse neuroblastoma and erythroleukemia cells. Cancer Lett 105: 233–239, 1996
Hu RH, Pegg AE: Rapid induction of apoptosis by deregulated uptake of polyamine analogues. Biochem J 328: 307–316, 1997
Tome ME, Fiser SM, Payne CM, Gerner EW: Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem J 328: 847–854, 1997
Hanauske-Abel HM, Slowinska B, Zagulska S, Wilson RC, Staiano-Coico L, Hanauske AR, McCaffrey T, Szabo P: Detection of a subset of polysomal mRNAs associated with modulation of hypusine formation at the G1–S boundary. Proposal of a role for eIF-5A in the onset of DNA replication. FEBS Lett 366: 92–98, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, Y., Kim, HK., Park, HE. et al. Effect of N 1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on endothelial cell growth, differentiation and apoptosis. Mol Cell Biochem 237, 69–76 (2002). https://doi.org/10.1023/A:1016535217038
Issue Date:
DOI: https://doi.org/10.1023/A:1016535217038