Abstract
Objectives: To assess the problems involved with the collection and interpretation of serial collected health related quality of life assessments in patients with malignant glioma.
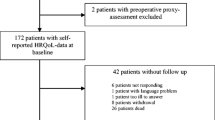
Patients and methods: One-hundred and fifty nine patients with malignant glioma from three Scottish neurosurgical centres in whom assessments of performance status, neurological impairment, mood, and quality of life had been recorded over a 6-month period were prospectively identified. The amount of missing data and the reasons for missing data were assessed. Characteristics of patients that were fully compliant with serial assessments were then compared with those that were not.
Results: Compliance with serial assessments (both patient and observer reported) was poor, dropping to less than 50% at 6 months. Observer reported measures showed a similar pattern of decline as patient reported measures. The largest single cause of missing data (approximately 70%) was due to administrative failure. Compliant patients were found to have a significantly greater probability of survival compared to non-compliant patients and were also found to be younger and fitter relative to the rest of the study population.
Conclusions: Studies utilising quality of life outcomes should give early consideration to minimising avoidable sources of missing data and recording the reasons for non-compliance. Quality of life studies basing conclusions on a complete case analysis should be wary of possible bias.
Similar content being viewed by others
References
ASCO:Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol 14(2): 671-679, 1996
Yung WKA, Prados MD, Yara-Tur, Rosenfeld SS, Brada M, Friedman HS Albright R, Olson J, Chang SM, O'Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA: Multicentre Phase II trial of Temozolamide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol 17(9): 2762-2771, 1999
Yung WKA, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA: A Phase II study of temozolamide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83(5): 588-593, 2000
Davies E, Hopkins A: Good Practice in the management of malignant cerebral glioma: clinical guidelines. Br J Neurosurg 11: 318-330, 1997
Clyde Z, Chataway SJ, Signorini D, Gregor A, Grant R: Significant change in tests of neurological impairment in patients with brain tumours. J Neuro-Oncol 39: 81-90, 1998
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Fletchner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan MY, Takeda F: The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365-376, 1993
Osboda D, Aaronson NK, Muller M, Sneeuw K, Hsu M-A, Yung WKA, Brada M, Newlands E: The development and psychometric validation of a brain cancer quality of life questionnaire for use in combination with general cancer specific questionnaires. Qual Life Res 5: 139-150, 1996
Hopwood P, Stephens RJ, Machin D: Approaches to the analysis of quality of life data: experiences gained from a Medical Research Council Lung Cancer Working Party palliative chemotherapy trial. Qual Life Res 3: 339-352, 1994
Machin D, Weeden S: Suggestions for the presentation of quality of life data from clinical trials. StatMed17: 711-724, 1998
Moinpour C, Lovato L: Ensuring the quality of quality of life data: the Southwest Oncology Group experience. Stat Med 17: 641-651, 1998
Simes JR, Greatorex V, Bebski VJ: Practical approaches to minimise problems with missing quality of life data. Stat Med 17: 641-651, 1998
Bernhard J, Cella DF, Coates AS, Fallowfield L, Ganz PA, Moinpour CM, Mosconi P, Osoba D, Simes J, Hurny C: Missing quality of life data in cancer clinical trials: serious problems and challenges. Stat Med 17: 517-532, 1998
Lindsey JK: Revealing Statistical Principles. Arnold, London, p 26, 1999
Curran D, Bacchi M, Schmitz S, Molenberghs G, Sylvester RJ: Identifying the types of missingness in quality of life data from clinical trials. Stat Med 17: 739-756, 1998
Kiebert GM, Curran D, Asronson NK: Quality of life as an endpoint in EORTC clinical trials. Stat Med 17: 561-569, 1998
Berhard J, Gusset H, Hurny C: Practical issues in quality of life assessment in multicentre trials conducted by the Swiss Group for Clinical Cancer Research. Stat Med 17: 633-639, 1998
Troxel AB, Fairclough DL, Curran D, Hahn EA: Statistical analysis of quality of life with missing data in cancer clinical trials. Stat Med 17: 653-666, 1998
Fairclough DL: Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med 16: 1197-1209, 1997
Fletcher A, Gore S, Jones D, Fitzpatrick R, Spiegelhalter D, Cox D: Quality of life measures in health care: II Design, analysis and interpretation. BMJ 305: 1145-1148, 1992
Fayers PM, Hopwood P, Harvey A, Girling DJ, Machin D, Stephens R: Quality of life assessment in clinical trials: guidelines and a checklist for protocol writers - The UK Medical Research Council experience. Eur J Cancer 33: 20-28, 1997
Staquet M, Berzon R, Osoba D, Machin D: Guidelines for reporting results of quality of life assessments in clinical trials. Qual Life Res 5: 496-502, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walker, M., Brown, J., Brown, K. et al. Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma. J Neurooncol 63, 179–186 (2003). https://doi.org/10.1023/A:1023900802254
Issue Date:
DOI: https://doi.org/10.1023/A:1023900802254